(通讯员 陈佶棠)8月21日,国际顶级化学综合期刊Accounts of Chemical Research (化学研究述评) (影响因子21.661) 在线发表了我校生命学院国家纳米药物工程技术研究中心杨祥良教授和李子福教授课题组的综述论文,题目为《肿瘤血管靶向智能纳米药物》 (Smart Nanotherapeutic Targeting of Tumor Vasculature),标志该课题组及合作团队在肿瘤血管靶向智能纳米药物取得系列成果。
高度紊乱的血管系统是恶性实体肿瘤的共性特征,肿瘤血管靶向治疗已成为肿瘤治疗领域的重要组成部分,多种肿瘤血管靶向药物也应运而生,有些(如贝伐珠单抗)已经获批上市。然而,目前用于肿瘤血管靶向治疗的小分子或抗体药物面临疗效低、毒副作用大等临床挑战。与小分子和抗体药物相比,智能纳米药物具备以下优势:第一,利用靶向配体分子(如多肽和抗体)对纳米药物的表面进行功能化修饰,纳米药物能够精确地靶向肿瘤血管内皮细胞,从而减少脱靶效应,提高抗肿瘤效果;其次,纳米载体可以同时携带多种抗肿瘤药物,实现时/空间精准同步抗肿瘤综合治疗;第三,可以将治疗药物和分子影像对比剂整合到一个纳米载体,实现肿瘤血管的诊疗一体化;第四,纳米药物可以实现肿瘤微环境刺激响应智能释药,定点清除肿瘤。因此,使用智能纳米药物靶向肿瘤血管治疗前景广阔。
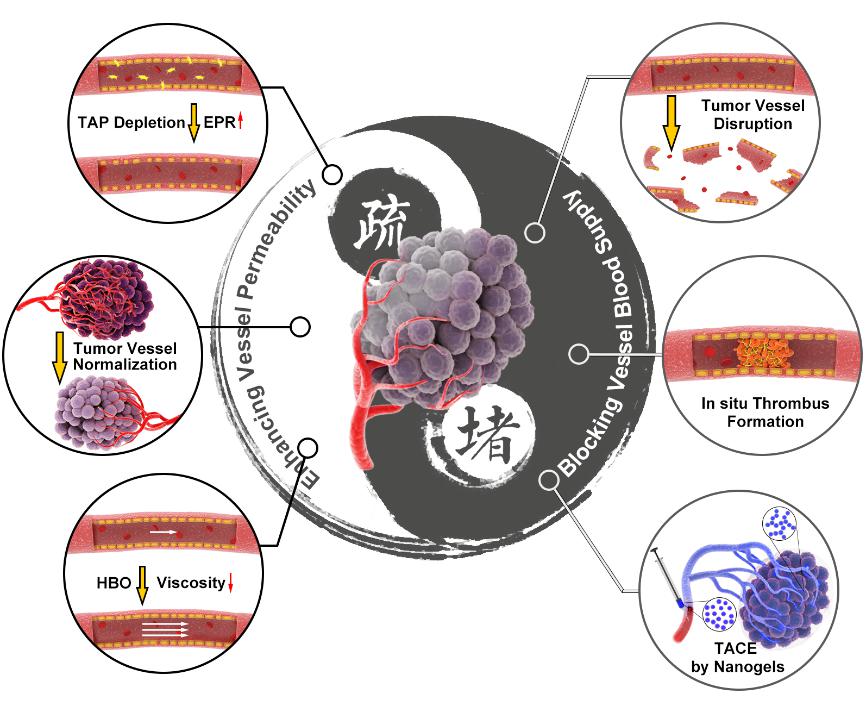
该综述论文基于生命学院杨祥良教授团队和国家纳米科学中心聂广军教授团队的前期工作,提出看似矛盾实则不矛盾的肿瘤血管堵与疏的调控策略。堵的目的是为了切断肿瘤细胞营养与氧气的供给,疏的目的是为了提高抗肿瘤药物的递送效率与疗效。杨祥良教授团队研制出一种温敏纳米凝胶实现肝癌高效与特异性栓塞,并提出使用高压氧治疗降低肿瘤血液黏度、增强血流灌注、克服肿瘤力学微环境屏障、提高纳米药物递送效率和抗肿瘤疗效。在肿瘤血管堵的调控策略方面,聂广军教授团队首次构筑出装载凝血酶的DNA纳米机器人实现肿瘤部位超选择性原位血栓生成、掐断肿瘤细胞营养与氧气的供给,发展了肿瘤部位酸响应纳米药物摧毁肿瘤补给血管。在肿瘤血管疏的调控策略方面,聂广军教授团队研制出肿瘤微环境高表达基质金属蛋白酶响应纳米药物选择性清除肿瘤相关血小板、提高肿瘤血管通透性与抗肿瘤药物瘤内富集量,构建了肿瘤部位酸响应纳米药物递送小干扰RNA敲除肿瘤血管上皮细胞关键蛋白、使肿瘤血管正常化。这6种肿瘤血管调控策略都取得极为显著的抗肿瘤疗效。
经导管动脉栓塞(TAE)是一种在医学影像设备的引导下,通过导管选择性地将栓塞剂注入靶动脉,以阻断靶动脉进行肿瘤治疗的技术。TAE被认为是一种革命性的肿瘤治疗方法,其疗效迅速,并发症发生率低,且易于与其它治疗方法结合。例如,TAE与化疗药物的结合,也被称为经导管动脉化疗栓塞(TACE),是不可切除肝细胞癌(HCC)一线姑息疗法的金标准治疗方案。但是,目前TAE和TACE临床疗效严重受限于血管栓塞剂。临床上广泛使用的固体栓塞剂(包括明胶海绵、聚乙烯醇微颗粒、海藻酸钙微球)和液体栓塞剂(包括无水酒精和碘油)都有明显的缺点:固体栓塞剂具备较好的栓塞性,可以栓塞肿瘤大血管但很难同时实现末梢血管栓塞;液体栓塞剂具备较好的流动性,可以栓塞肿瘤末梢血管但面临栓塞强度较低的问题。这些栓塞剂无法同时兼顾栓塞性和流动性,栓塞不足常引起肿瘤血管再通和肿瘤内血液循环,最终导致肿瘤的复发和转移。受血液凝固级联反应的启发,杨祥良教授和赵彦兵教授课题组与华中科技大学附属协和医院郑传胜教授合作2011年基于聚(N-异丙基丙烯酰胺-甲基丙烯酸丁酯)(PIB)研发了一种智能纳米凝胶。该纳米凝胶展示出独特的温度触发的溶胶-凝胶相转变过程,可实现肝癌大血管和末梢血管永久性同步栓塞。这种新型血管栓塞剂PIB纳米凝胶有效的解决了介入治疗流动性和栓塞性的矛盾。团队研制拥有自主知识产权、效果显著优于现有国内外产品的新型肝癌介入栓塞材料,近10年在Advanced Functional Materials,Theranostics,Journal of Controlled Release等高水平杂志上发表论文近20篇。更重要的是,该团队掌握核心材料的小试、中试及放大生产的工艺研究、验证及质量控制的关键技术,有望打破我国肝癌介入治疗严重依赖进口碘油的卡脖子现状。
为促进侵袭与转移,肿瘤发展出一种独特的血管新生模式,通过在已有的毛细血管上萌芽形成新的血管。这些新生血管迂曲、渗漏,结构完整性差,内皮细胞间隙大,平滑肌细胞缺失,周细胞和基底膜覆盖不全。与正常的血管系统不同,肿瘤血管中的血流并不总是沿着固定的、单向的轨迹流动;并非所有的血管都灌注良好,血流可能在短时间内通过同一根血管走不同的路径,甚至在相反的方向流动。肿瘤血管结构和功能性异常导致蛇形血流和灌注不良,形成一种独特的微环境(肿瘤微环境;TME),表现为乏氧、高凝和免疫抑制。杨祥良教授和李子福教授课题组巧妙的利用临床常用的高压氧治疗降低肿瘤血管中血液黏度、提高血流灌注、改善肿瘤微环境、显著增强纳米药物抗肿瘤疗效,相关研究工作发表在Advanced Science。
化学研究述评上的论文主要是阐述自己在某一方面的系统性工作。李子福教授为该论文第一作者,杨祥良教授为共同通讯作者,共同通讯作者还包括国家纳米科学中心李素萍教授和聂广军教授。华中科技大学为第一作者单位。该论文得到了国家重点研究计划项目,国家自然科学基金以及华中科技大学学术前沿青年团队的资助。
论文链接:https://pubs.acs.org/doi/10.1021/acs.accounts.9b00283
(Correspondent Jitang Chen) On August 21st, a review paper entitled “Smart Nanotherapeutic Targeting of Tumor Vasculature” has been published on Accounts of Chemical Research (American Chemical Society). This review is contributed by the team of Prof. Xiangliang Yang and Prof. Zifu Li from College of Life Science & Technology, HUST and National Engineering Research Center for Nanomedicine, symbolizing this research team and the collaborative research team have accomplished a series of achievements in leveraging smart nanotherapeutics modulating tumor vasculature.

Highly chaotic vascular system is a common feature of malignant solid tumors. Tumor vascular targeting therapy has therefore become an important part of cancer therapy. Various tumor vascular targeting drugs have emerged, with some, for instance bevacizumab, being approved for clinical practice. Nonetheless, the existing small molecule- or antibody-based drugs for tumor vessel targeted therapy do not well satisfy clinical application criteria such as favorable tumor accumulation and low toxicity. In contrast, nanotherapeutics display attractive properties for improving drug therapeutic efficacy or revolutionizing currently vascular targeting strategies. First, by functionalizing the surfaces of nanotherapeutics with targeting ligand molecules (e.g., peptides, aptamers and antibodies), nanotherapeutics can precisely target and bind tumor endothelial cells, thus reducing adverse, off-target effects and improving antitumor efficacy. Second, nanocarriers can simultaneously carry multiple therapeutic agents to affect combined therapy, precisely synchronizing both the temporal and spatial antitumor attack. Third, nanotherapeutics can integrate therapeutic drugs with molecular imaging agents into a single platform to achieve theranostics in tumor vessels. Forth, smart nanotherapeutics can achieve triggered drug release in response to tumor microenvironment stimuli. Therefore, the use of smart nanotherapeutics targeting tumor vasculature has broad prospects.
Based on previous work of Professor Xiangliang Yang and Professor Guangjun Nie from National Center for Nanoscience and Technology of China, this review paper proposes seemingly contradictory strategies for the regulation of tumor vasculature: blocking and dredging. The purpose of blocking is to cut off the supply of nutrition and oxygen to cancer cells, whereas the aim of dredging is to improve the delivery efficiency and efficacy of antitumor drugs. The team of Professor Xiangliang Yang developed a smart polymeric nanogel for permanent and peripheral embolization of liver tumors and leveraged hyperbaric oxygen (HBO) therapy to decrease the viscosity and promote tumor perfusion of a nanomedicine. To selectively block tumor blood supply and starve the tumor to death, the team of Professor Guangjun Nie developed a thrombin loaded DNA nanorobots and tumor acidity responsive nanoparticle. In addition, this team constructed a hybrid nanoparticle to selectively deplete tumor associated platelets and prepared tumor acidity responsive nanotherapeutics to correct tumor vasculature abnormalities, for the purpose of enhanced tumor vessel permeability. These six strategies have all achieved remarkable anti-tumor effects.
Transcatheter arterial embolization (TAE) is a technique of selectively injecting embolic agents through a catheter to a target artery under the guidance of medical imaging devices to obstruct the target artery for cancer therapy. Considered a revolutionary treatment, TAE produces rapid effects, while incurring a low incidence of complications, and is easy to combine with other therapies. For instance, the combination of TAE with chemotherapeutic drugs, also known as transcatheter arterial chemoembolization (TACE), the gold standard, first-line palliative therapy for unresectable hepatocellular carcinoma (HCC) which was shown to have the largest concentration case in Asia. However, the clinical efficacy of TAE and TACE is severely limited by vascular embolic agents. A wide range of solid and liquid embolic agents are used clinically for TAE/TACE against HCC, including gelatin sponges, polyvinyl alcohol (PVA) particles, calcium alginate microspheres, absolute alcohol and Lipiodol. However, all these materials suffer from overt shortcomings. Insufficient embolization often induces vascular recanalization and collateral circulation in the tumor, ultimately leading to tumor recurrence and metastasis. Inspired by the changes in blood from a liquid state to a solid-like gel state in the conversion of soluble fibrinogen into crosslinked fibrin clots, the team of Professor Xiangliang Yang and Professor Yanbing Zhao, together with Professor Chuansheng Zheng from Union Hospital of Huazhong University of Science and Technology, developed a smart poly (N-isopropylacrylamide-co-butyl methylacrylate) (PIB) nanogel, which displays an intriguing temperature-triggered sol-gel phase transition, for permanent and peripheral embolization of liver tumors. This novel blood-vessel-embolic PIB nanogel largely resolves the dilemma of flowability and embolization in TAE/TACE therapy. The team has developed a novel type of interventional embolic material, with independent intellectual property rights, being superior to the existing products at home and abroad. In recent 10 years, nearly 20 papers have been published by this team in top peer-reviewed journals, such as Advanced Functional Materials, Theranostics, Journal of Controlled Release, to name a few. More importantly, the team has developed the key technologies of small-scale, pilot-scale and enlarged production of core materials, such as process research, verification and quality control.
For progression and metastasis to occur, tumors develop a unique neovascularization paradigm, in which new blood vessels form by sprouting on pre-existing capillaries1. The newly formed vessels are tortuous and leaky, with a lack of structural integrity, containing large gaps between endothelial cells, missing smooth muscle cells, and exhibiting incomplete coverage by pericytes and basement membrane. Unlike normal vasculature, the blood flow in tumors does not always follow a constant, unidirectional track; not all vessels are perfused well, and the blood flow may take varied paths and even flow in opposed directions via the same vessel over a short period of time. The structural and functional vessel abnormalities contribute to a prothrombotic state, serpentine blood flow, and poor perfusion, thereby shaping a distinctive microenvironment (tumor microenvironment; TME) generally characterized by hypoxia, hypercoagulation and immunosuppression. The research team of Professor Yang Xiangliang and Professor Li Zifu skillfully leveraged the commonly used hyperbaric oxygen therapy in clinic to reduce blood viscosity, improve blood perfusion, modulate tumor hypoxia and mechano-microenvironment, and significantly boost the anti-tumor efficacy of nanomedicine Doxil. The related research work was published in Advanced Science.
Review papers on the Accounts of Chemical Research mainly expound the systematic work of oneself in one aspect. Professor Zifu Li is the first author of this review paper, Professor Xiangliang Yang is the corresponding author, and the corresponding authors also include Professor Suping Li and Professor Guangjun Nie from National Center for Nanoscience and Technology of China. The first author affiliation is Huazhong University of Science and Technology. This review paper is supported by grants from the National Key Research and Development Program of China, National Science Foundation of China, and the Program for HUST Academic Frontier Youth Team.
The paper link: https://pubs.acs.org/doi/10.1021/acs.accounts.9b00283
The paper citation: Zifu Li, Chunzhi Di, Suping Li, Xiangliang Yang, Guangjun Nie. Smart Nanotherapeutic Targeting of Tumor Vasculature. Accounts of Chemical Research (2019), DOI: 10.1021/acs.accounts.9b00283