(Correspondents: Xianping Wang, Jihong Gong) On July 9th, a research paper entitled "Munc13 activates the Munc18-1/syntaxin-1 complex and enables Munc18-1 to prime SNARE assembly" by Professor Cong Ma’s research group in college of life science and technology of Huazhong University of Science and Technology was published in The EMBO Journal.
(This image is from the internet)
Synaptic secretion is the material basis of information transmission between neurons. Synaptic vesicles loaded with neurotransmitters arrive at the active zone on the presynaptic membrane via complicated transport process, and undergo docking and priming process to be in a state of ready release. Triggered by Ca2+ signals, the rapid release of synaptic vesicles occurs within a millisecond time range. The core proteins that mediate synaptic vesicle fusion with presynaptic membrane are SNARE proteins (including syntaxin-1 and SNAP-25 on the presynaptic membrane, and synaptobrevin-2 on the vesicle). The three SNAREs assemble into SNARE complex to bring the two membranes into close proximity and drive membrane fusion. The landmark event of vesicle priming is the formation of SNARE complex which requires complex and exquisite regulations by multiple regulatory proteins. Among them, Munc18-1 and Munc13 are particularly important.
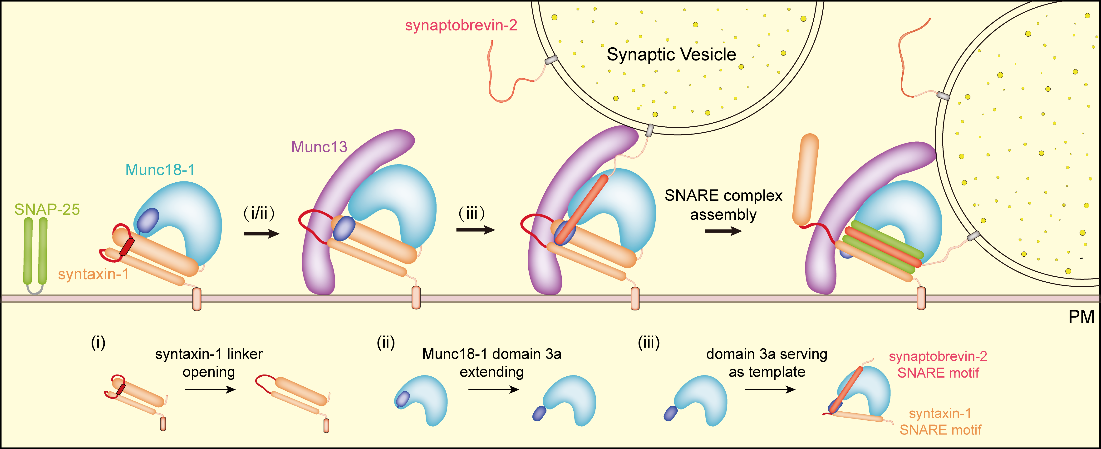
By the combined use of methods of biochemistry, cell biology, electrophysiology, single-molecule FRET and X-ray crystalology, we found Munc13-1 catalyzes the open of syntaxin-1 linker region via an interaction with Munc18-1 domain 3a, leading to the conformational change of domain 3a from bend to extension, thus activating Munc18-1/syntaxin-1 complex. The domain 3a adopts extended conformation binding SNARE motifs of synaptobrevin-2 and syntaxin-1 to serve as a template for SNARE assembly. This study elucidated the molecular mechanism of Munc13-1 activating Munc18-1/Syntaxin-1 complex in multiple perspectives, and demonstrated the essential role of activated Munc18-1 domain 3a in synaptic secretion.
This paper expands the view of Professor Cong Ma's research group in the field of neurotransmitter secretion mechanism [Science (2013), Nature Structural & Molecular Biology (2015), The EMBO Journal (2017), Nature Communications (2019), and Cell Reports (2019)]. The results further elucidated the molecular mechanism and the crucial role of Munc18 and Munc13 family proteins in regulating synaptic secretion.
Professor Cong Ma from Huazhong University of Science and Technology is the corresponding author of this paper, the PhD candidates Xianping Wang, Jihong Gong and Le Zhu from the Synaptic Secretion Laboratory of School of Life Science and Technology are the co-first authors of this paper. The postdoctors Shen Wang, Xiaoyu Yang, the PhD candidate Yuanyuan Xu from Synaptic Secretion Laboratory of School of Life Science and Technology, and the Professor Xiaofei Yang from South-Central University for Nationalities participated in the work. This work was supported by the grants from the National Natural Science Foundation of China, the National Key Basic Research Program of China, and funds from Huazhong University of Science and Technology.
Contributor: Synaptic Secretion Laboratory of Professor Cong Ma's Group