(Correspondent Qiang Wang) On Feb 21st, a research paper entitled “Influence of Linkers within Stimuli-Responsive Prodrugs on Cancer Therapy: A Case of Five Doxorubicin Dimer-Based Nanoparticles” has been published on Chemistry of Materials, which proposed a novel strategy to construct potent stimuli-responsive prodrugs. This work is contributed by the team of Prof. Zifu Li and Prof. Xiangliang Yang from College of Life Science & Technology and National Engineering Research Center for Nanomedicine, HUST.
Prodrugs are bio-reversible derivatives of drug molecules that undergo an enzymatic and/or chemical transformation in vivo to release the active parent drugs. In both drug discovery and development, prodrugs have become a classic tool for the discovery of new and promising chemical entities. In recent years, stimuli-responsive prodrugs, including small molecular-drug conjugates (SMDC), peptide-drug conjugates (PpDC), polymer-drug conjugates (PDC), antibody-drug conjugates (ADC) and emerging aptamer-drug conjugates (ApDC), have been flourishing for the treatment of numerous malignancies.
Linkers are an essential component in stimuli-responsive prodrugs and determine the release of the drug at the pathological site. For treatment of malignant tumors, commonly used cleavable linkers include: hydrazone bonds, disulfide (selenium) bonds, boronic esters, thioketal, azobenzene, peptide substrates, etc. Their cleavage is mainly dependent on tumor microenvironment (TME), including: acidity, high levels of glutathione (GSH) and reactive oxygen species (ROS), and high expression of enzymes (e.g., cathepsins and matrix metalloproteinases). However, given that solid malignancies have so many abnormalities and disparate pathophysiological features, there is a compelling requirement to seek the best stimuli-responsive linker for potent cancer therapy against various solid malignancies.
Based on the background mentioned above, we compared the antitumor efficacy of five stimuli-responsive DOX dimeric prodrug NPs in orthotopic 4T1 and subcutaneous H22 tumors. These prodrugs were linked with stable carbon chain, trace amide-disulfide, and traceless carbamate-disulfide, borate ester, and dimethylmaleamide linkers and could self-assemble into NPs without any carrier. With these self-assembled NPs, we demonstrated that traceless carbamate-disulfide linker exhibited superiority than non-cleavable carbon chain and trace amide-disulfide linker. But no prodrug NPs exhibited superior antitumor efficacy and cleavage rate in tumors of TNBC and HCC. We further revealed different cancer cells exhibited distinctive capacity to maintain endogenous chemical species while inter-tumoral heterogeneity hampered the availability of prodrug NPs with broad spectrum superiority.
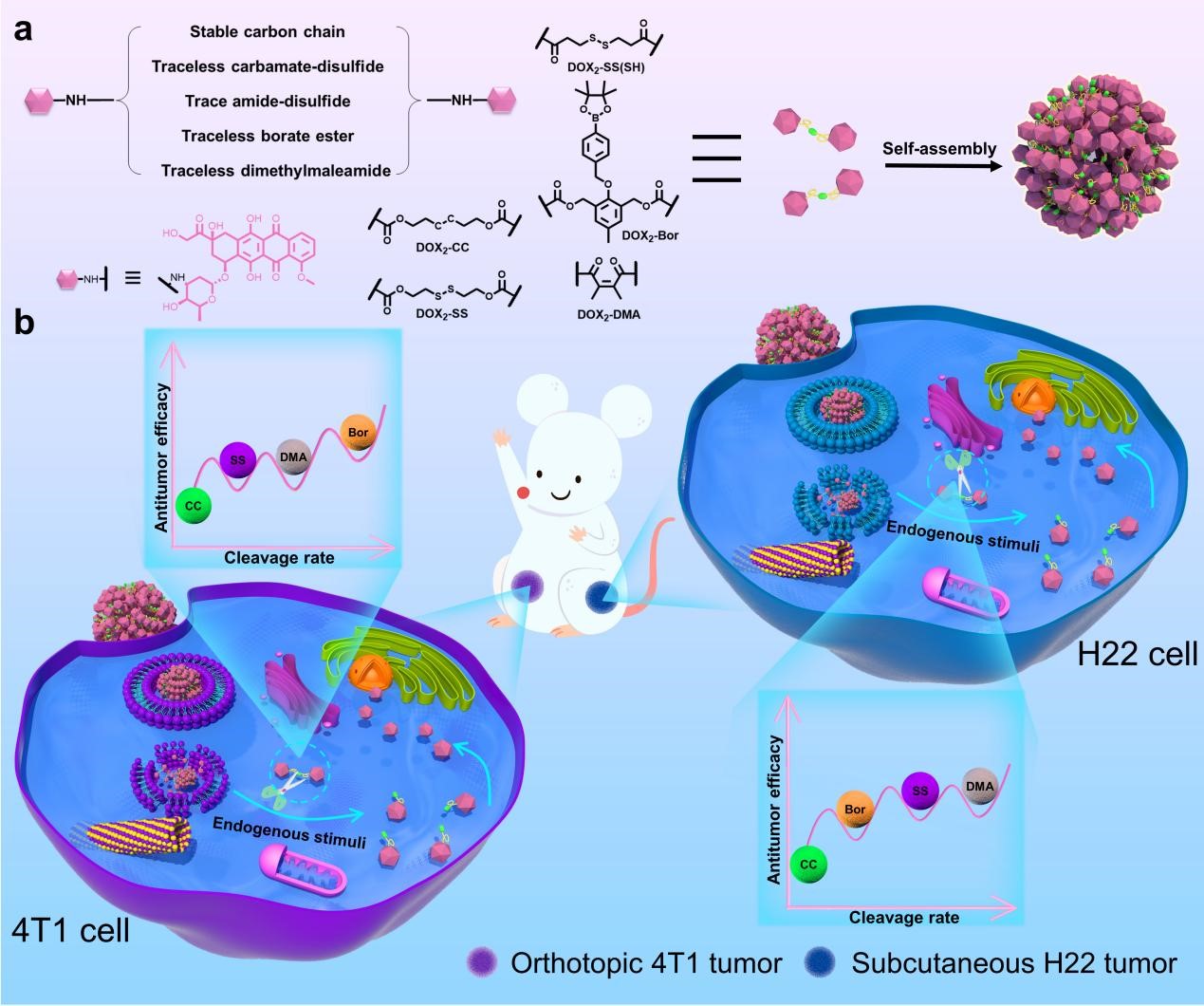
Based on above results, we proposed that a precise and traceless linker should be pursued for construction of potent stimuli-responsive prodrugs.Precise refers to fully consideration of inter-tumoral heterogeneity of TME while traceless pledges release of parent drugs, altogether ensuring that native drugs could reach specified subcellular organelle for potent antitumor efficacy.
Ph.D. candidates Qiang Wang, Chong Wang, and Shiyou Li, from College of Life Science & Technology, HUST contributed equally to this work. This work was financially supported by grants from the National Key Research and Development Program of China (2020YFA0211200, 2020YFA0710700, and 2018YFA0208900), the National Science Foundation of China (31972927, 82172757), the Scientific Research Foundation of HUST (3004170130), the Program for HUST Academic Frontier Youth Team (2018QYTD01), and the HCP Program for HUST.
Article Link: https://pubs.acs.org/doi/10.1021/acs.chemmater.1c03346