(Correspondent Jitang Chen) On August 21st, a review paper entitled “Smart Nanotherapeutic Targeting of Tumor Vasculature” has been published on Accounts of Chemical Research (American Chemical Society). This review is contributed by the team of Prof. Xiangliang Yang and Prof. Zifu Li from College of Life Science & Technology, HUST and National Engineering Research Center for Nanomedicine, symbolizing this research team and the collaborative research team have accomplished a series of achievements in leveraging smart nanotherapeutics modulating tumor vasculature.
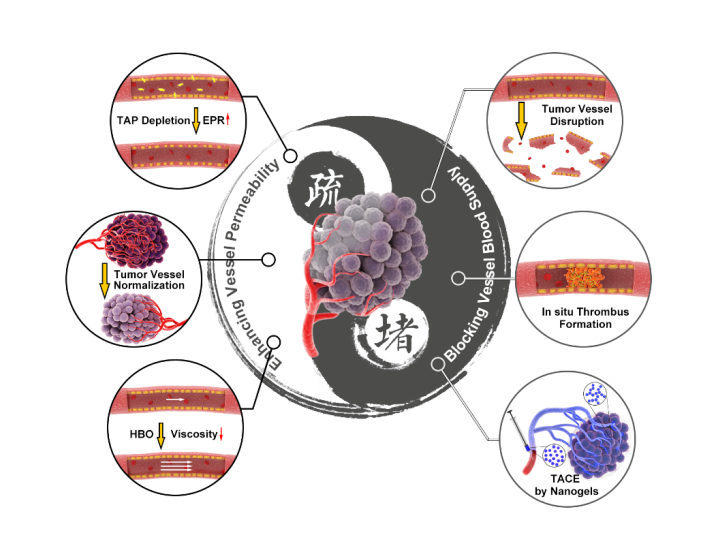
Highly chaotic vascular system is a common feature of malignant solid tumors. Tumor vascular targeting therapy has therefore become an important part of cancer therapy. Various tumor vascular targeting drugs have emerged, with some, for instance bevacizumab, being approved for clinical practice. Nonetheless, the existing small molecule- or antibody-based drugs for tumor vessel targeted therapy do not well satisfy clinical application criteria such as favorable tumor accumulation and low toxicity. In contrast, nanotherapeutics display attractive properties for improving drug therapeutic efficacy or revolutionizing currently vascular targeting strategies. First, by functionalizing the surfaces of nanotherapeutics with targeting ligand molecules (e.g., peptides, aptamers and antibodies), nanotherapeutics can precisely target and bind tumor endothelial cells, thus reducing adverse, off-target effects and improving antitumor efficacy. Second, nanocarriers can simultaneously carry multiple therapeutic agents to affect combined therapy, precisely synchronizing both the temporal and spatial antitumor attack. Third, nanotherapeutics can integrate therapeutic drugs with molecular imaging agents into a single platform to achieve theranostics in tumor vessels. Forth, smart nanotherapeutics can achieve triggered drug release in response to tumor microenvironment stimuli. Therefore, the use of smart nanotherapeutics targeting tumor vasculature has broad prospects. Based on previous work of Professor Xiangliang Yang and Professor Guangjun Nie from National Center for Nanoscience and Technology of China, this review paper proposes seemingly contradictory strategies for the regulation of tumor vasculature: blocking and dredging. The purpose of blocking is to cut off the supply of nutrition and oxygen to cancer cells, whereas the aim of dredging is to improve the delivery efficiency and efficacy of antitumor drugs. The team of Professor Xiangliang Yang developed a smart polymeric nanogel for permanent and peripheral embolization of liver tumors and leveraged hyperbaric oxygen (HBO) therapy to decrease the viscosity and promote tumor perfusion of a nanomedicine. To selectively block tumor blood supply and starve the tumor to death, the team of Professor Guangjun Nie developed a thrombin loaded DNA nanorobots and tumor acidity responsive nanoparticle. In addition, this team constructed a hybrid nanoparticle to selectively deplete tumor associated platelets and prepared tumor acidity responsive nanotherapeutics to correct tumor vasculature abnormalities, for the purpose of enhanced tumor vessel permeability. These six strategies have all achieved remarkable anti-tumor effects.
Professor Zifu Li is the first author of this review paper, Professor Xiangliang Yang is the corresponding author, and the corresponding authors also include Professor Suping Li and Professor Guangjun Nie from National Center for Nanoscience and Technology of China. The first author affiliation is Huazhong University of Science and Technology. This review paper is supported by grants from the National Key Research and Development Program of China, National Science Foundation of China, and the Program for HUST Academic Frontier Youth Team.
The paper link: https://pubs.acs.org/doi/10.1021/acs.accounts.9b00283
The paper citation: Zifu Li, Chunzhi Di, Suping Li, Xiangliang Yang, Guangjun Nie. Smart Nanotherapeutic Targeting of Tumor Vasculature. Accounts of Chemical Research (2019), DOI: 10.1021/acs.accounts.9b00283