On Dec 20, 2020, a research article entitled “A versatile strategy for improving phototherapeutic efficacy on deep-sited tumor by tissue optical clearing technique” has been published in Nano Today (IF: 16.907), which was performed by Prof. Xiangliang Yang and Prof. Yanbing Zhao’s group from National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology.
Cancer is one of the deadly diseases that seriously endanger human health. Traditional cancer treatment methods such as surgery, chemotherapy and radiotherapy will lead to severe toxic and side effects, drug resistance and metastasis of tumors. Compared with the traditional cancer treatment methods, phototherapies (such as photothermal/photodynamic therapy) based on multifunctional nanomaterials exhibits obvious advantages, such as non-invasive, remote manipulation, high temporal and spatial resolution and low toxic and side effects. In recent years, it has achieved great progress in treatment of superficial tumors clinically. However, due to the high barrier action of human tissues to light, these efficient phototherapy modes are difficult to achieve effective treatment on deep-sited tumors. Although some longer-wave sources (such as far-infrared light, microwave, radiofrequency field, etc.) can improve tissue penetration, the mismatch of refractive index between interstitial fluid and tissue substance still hinder these light/electromagnetic waves reach to deep-sited tumors. Therefore, how to fundamentally improve the tissue penetration depth of the light and subsequently enhance the phototherapeutic potency to deep-sited tumors remains urgent.
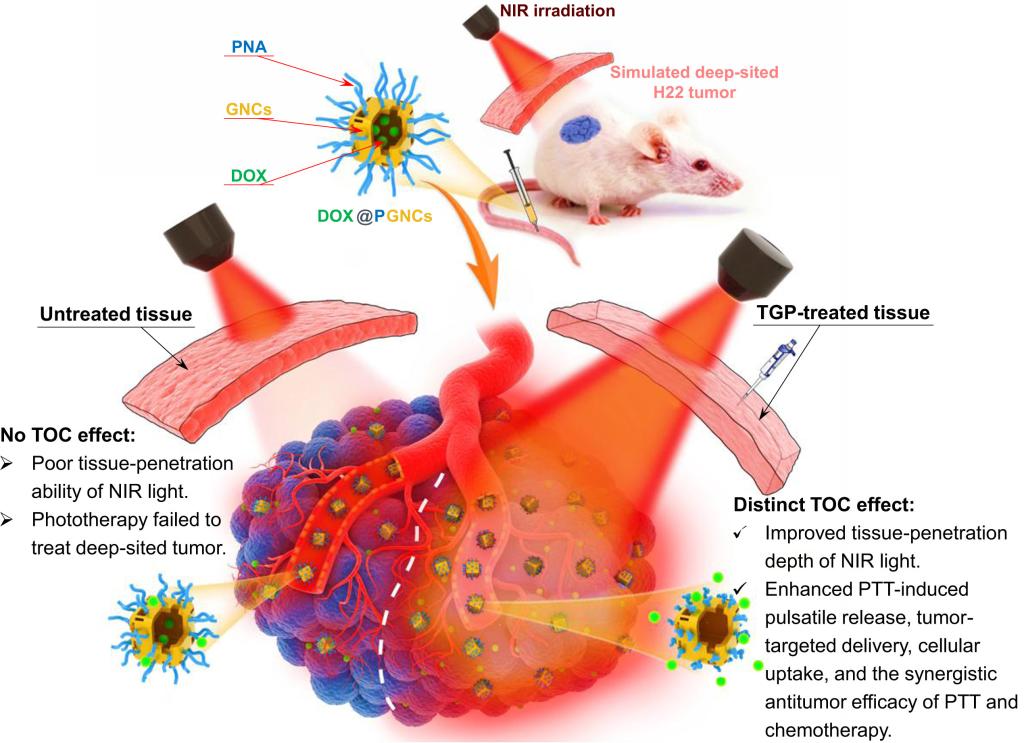
In order to solve this problem, this study developed a highly effective and biocompatible tissue optical clearing agent (TGP) for combining tissue optical clearing (TOC) technique with tumor phototherapy to improve phototherapeutic efficacy on deep-sited tumors. Experiments on the ex vivo pig skin and the skin of abdomen of live rats showed that the transmittance of skin increased by four times after 30 min of TGP treatment. Doxorubicin-loaded gold nanocages (DOX@PGNCS) were synthesized as photothermo-chemotherapy nano-agents. Tumor inhibition experiments on the deep-sited tumor (simulated by covering subcutaneous tumor with ex vivo pig skin) showed that TGP pre-treatment could significantly boost the photothermo-chemotherapy effect of DOX@PGNCS, that was after 14 days treatment, the tumor volume was only 38% of that without TGP pre-treatment. Furthermore, TOC technique is easy to be "reversibly erased" with saline to avoid unnecessary adverse reactions. TOC technique can also effectively enhance photothermally-directed targeted tumor delivery, significantly increasing the tumor accumulation of DOX@PGNCS, thus achieving highly selective tumor photothermal ablation and chemotherapy. To sum up, the TOC technique is expected to fundamentally solve the tissue penetration depth problem of therapeutic laser, providing a new perspective for the application of various phototherapy modes (such as photothermal therapy and photodynamic therapy) in the treatment of deep-sited tumors.
Prof. Yanbing Zhao is the co-corresponding author. Hao Zhao is the co-first author. Jianshan Xu, Jiangshan Wan and Wenjing Huang are involved in the study. This research is supported by the National Key Research and development Program and the National Natural Science Foundation of China.