On April 1st, 2021,Associate Prof. Yan Zhang and Prof. Yanhong Zhu from College of Life Science & Technology and National Engineering Research Center for Nanomedicine published a research article entitled "Transformable Nanosensitizer with Tumour Microenvironment‐Activated Sonodynamic Process and Calcium Release for Enhanced Cancer Immunotherapy" in Angewandte Chemie International Edition, an internationally prestigeous chemistry journal of Wiley.
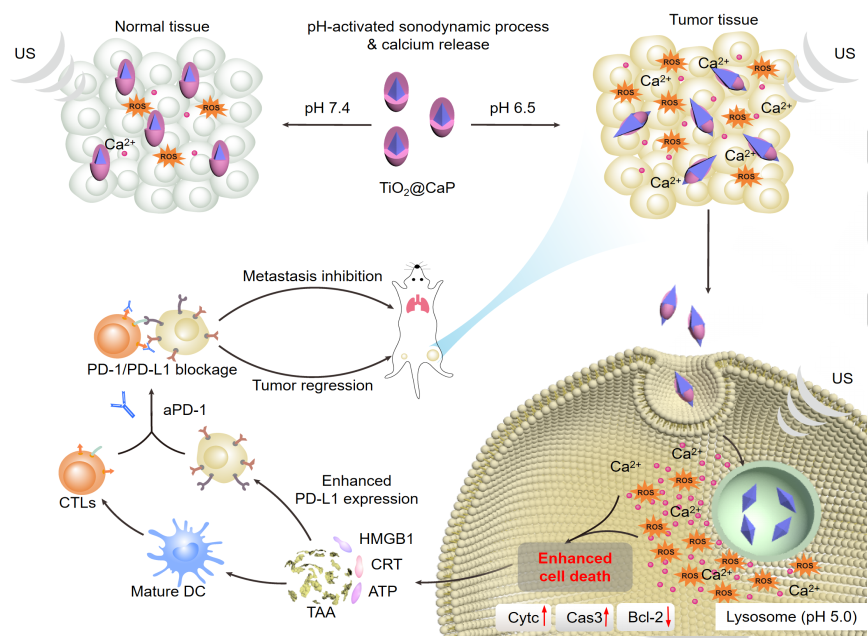
Sonodynamic therapy (SDT) emerges as a promising site-specific cancer therapeutic modality to treat solid tumors due to the unique features including operational feasibility, non-invasiveness and more importantly, high tissue penetration depth (up to ~ 10 cm). It utilizes sonosensitizers to generate cytotoxic reactive oxygen species (ROS) and induce oxidative damages towards cancer cells upon low-intensity ultrasound activation. To improve the biodistribution and therapeutic performance for small molecular sonosensitizers, polymeric and nanoparticulate sonosensitizers includingAu-MnO, Au-TiO2,MnWOx, TiO1+x[9], graphene, metal-organic-framework, Pt-CuS, etc. have been constructed. However, these sonosensitizers often have “always on” pharmacological activities regardless of the disease microenvironment, resulting in limited therapeutic selectivity and potential off-target toxicity to the healthy tissues.
To improve its anti-tumor efficacy, we report a transformable core-shell nanosonosensitizer (TiO2@CaP) that not only reinvigorates the ROS generation but also dissolves its CaP shell to release Ca2+ in acidic tumor microenvironment (TME) under ultrasound activation. Thus, TiO2@CaP acts as the smart nanosonosensitizer that specifically induces mitochondrial dysfunction via overloading intracellular Ca2+ ions to synergize with sonodynamic process in TME. Such a synergetic effect of TME-activated sonodynamic ROS generation and Ca2+ release is revealed to substantially enhance immunogenic cell death, resulting in enhanced T cell recruitment and infiltration into the immunogenetic cold tumor (4T1). In conjunction with checkpoint blockade therapy (anti-PD 1), TiO2@CaP-mediated sonodynamic therapy elicits systemic anti-tumor immunity, leading to regression of non-treated distant tumors and inhibition of lung metastasis. This work thus paves the way to develop “smart” TME-activatable sonosensitizer with tempospatial control over antitumor responses.
The Ph.D. candidate, Jingzhao Huang, graduate studuents, Xuan Tan and Yiqian Wang from College of Life Science & Technology are co-first authors of this article. Associate Prof. Yan Zhang, Prof. Yanhong Zhu from College of Life Science & Technology, Huazhong University of Science and Techonology and Prof. Pu Kanyi from Nanyang Technological University, Singapore are the corresponding authors.