On May 24, 2021, a research paper entitled “Biomimetic Sonodynamic Therapy-Nanovaccine Integration Platform Potentiates Anti-PD-1 Therapy in Hypoxic Tumors” has been published online in Nano Today(IF=16.907). This paper is completed by the team of Professors Xiangliang Yang and Lu Gan from National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology (HUST).
Immune checkpoint blockade (ICB) therapy, such as anti-programmed death receptor 1 (PD-1) antibody, represents a new breakthrough in clinical application for malignant cancer. However, the durable responses generated by single-agent therapy are still low due to the hypoxia and inadequate T cell infiltration in solid tumors. Therefore, the combination of other therapeutic modalities which can relieve tumor hypoxia and induce T cell infiltration into tumors is an optimal strategy for improved therapeutic outcomes of anti-PD-1 antibody.
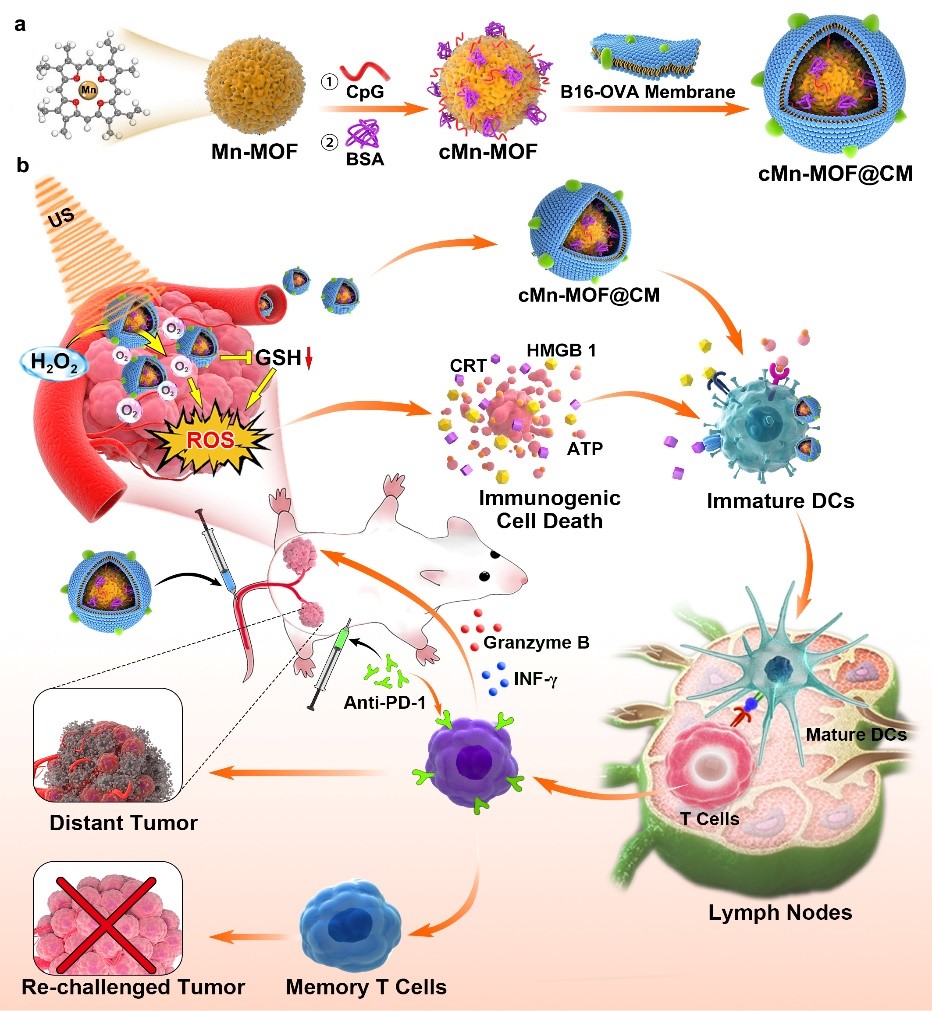
Sonodynamic therapy (SDT) holds great prospect in solid tumor therapy by using the ultrasound with high tissue-penetrating capability to penetrate into the tumor tissues and activating the sonosensitizer to produce reactive oxygen species and kill tumor cells. However, tumor microenvironment, such as hypoxia and high glutathione (GSH), severely influences the therapeutic effects of SDT. Here we design a sonodynamic therapy (SDT)-nanovaccine integration platform (cMn-MOF@CM) constructed by binding a manganese porphyrin-based metal-organic frameworks (Mn-MOF) with an immune adjuvant CpG and then coating with cell membranes derived from ovalbumin (OVA)-overexpressing melanoma B16 cells. cMn-MOF@CM effectively targets to tumor tissues of B16-OVA-tumor bearing mice, catalyzes tumor-overexpressed H2O2 to generate O2 for tumor hypoxia relief, and decreases intracellular GSH content, generating strong SDT effects in hypoxic tumors. The tumor-associated antigens both in situ derived from SDT and OVA exhibit vaccine-like functions together with the immune adjuvant CpG, eliciting a strong tumor-specific immune response by promoting DC maturation and T cell activation. Importantly, the combination of cMn-MOF@ CM-triggered SDT and anti-PD-1 antibody induces stronger systemic immune response and long-term immunological memory function to prevent tumor recurrence and metastasis. These findings reveal that cMn-MOF@CM may be a promising candidate for combining SDT and nanovaccine to improve the efficacy of anti-PD-1 antibody in solid tumors.
The corresponding authors of this work are Professors Lu Gan and Xiangliang Yang from College of Life Science and Technology, HUST. Ph. D student Guiting Zhan from College of Life Science and Technology, HUST, is the first author of this work. This work is supported by National Key R&D Program of China and National Natural Science Foundation of China.
Link:https://www.sciencedirect.com/science/article/pii/S1748013221001201