(Correspondent Wang Xiaoxian) On March 17th, a research paper entitled "Hyperbaric oxygen regulates tumor mechanics and augments Abraxane and gemcitabine antitumor effects against pancreatic ductal adenocarcinoma by inhibiting cancer-associated fibroblasts" has been published on Nano Today, which revealed the mechanism of hyperbaric oxygen enhancing the antitumor and anti-metastasis effect of clinical first-line therapy, and put forward a novel strategy for the treatment of pancreatic cancer. This work is contributed by the team of Prof. Zifu Li and Prof. Xiangliang Yang from College of Life Science & Technology and National Engineering Research Center for Nanomedicine, HUST.
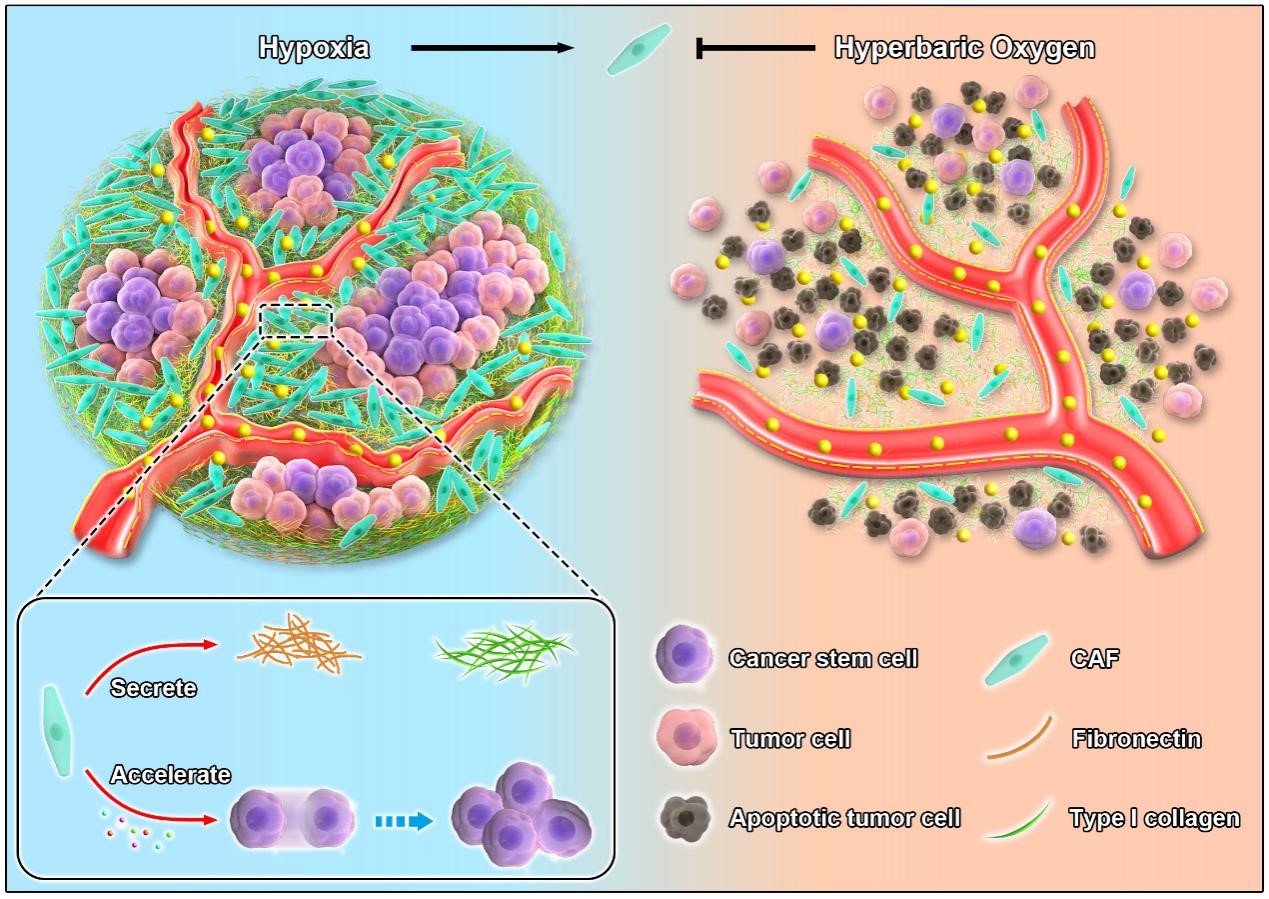
Pancreatic cancer is a highly malignant tumor of the digestive system, which is known as the "king of cancer" for its “indetectable, metastatic and intractable” properties. The existing clinical treatment of pancreatic cancer does n’t work effectively, and the 5-year survival rate is less than 10%. It’s urgently needed to develop other efficient treatments to benefit more patients with pancreatic cancer. The main obstacle limiting clinical efficacy of pancreatic cancer therapy is the abnormal mechanical microenvironment, in which cancer-associated fibroblasts (CAFs) play a key role. The proteins secreted by cancer-associated fibroblasts are the main components of dense extracellular matrix in pancreatic cancer. Together with cancer-associated fibroblasts, they increase tumor solid stress and mechanical stiffness, compress blood vessels and lymphatic vessels at the tumor site, and reduce tumor blood supply, thus reducing the antitumor effect of nanomedicine via tumor hypoxia and delivery blockade at the same time. In addition, a variety of cytokines secreted by cancer-associated fibroblasts create a cancer stem cells (CSCs) niche, which accelerates drug resistance, recurrence and metastasis of pancreatic cancer. Although consensus has been reached that both cancer-associated fibroblasts and hypoxia play crucial roles in tumor progression, little do we know about the regulatory mechanism of hypoxia on cancer-associated fibroblasts. The difficulty of elucidating the relationship between hypoxia and cancer-associated fibroblasts lies in how to relieve hypoxia effectively and specifically in pancreatic cancer.
In this study, hyperbaric oxygen, which is widely used in clinic, was utilized to overcome hypoxia in pancreatic cancer, and it was revealed for the first time that hyperbaric oxygen inhibited cancer-associated fibroblasts by alleviating hypoxia. In consequence, hyperbaric oxygen brough two benefits to pancreatic cancer treatment. On the one hand, cancer-associated fibroblasts inhibited by hyperbaric oxygen decreased the deposition of extracellular matrix notably, reduced tumor stiffness, normalized tumor vessels, and increased the accumulation, penetration as well as cellular uptake of nanomedicine in the tumor site. On the other hand, hyperbaric oxygen eliminated cancer stem cells effectively by regulating cancer stem cells niche mediated by cancer-associated fibroblasts. On the basis of the two mechanisms, hyperbaric oxygen combined with Abraxane and gemcitabine enhanced the antitumor and anti-metastasis efficacy of pancreatic ductal adenocarcinoma, thus providing a new strategy for pancreatic cancer treatment, that is, hyperbaric oxygen + Abraxane + gemcitabine.
In this study, hyperbaric oxygen was combined with clinical first-line treatment of pancreatic cancer for the first time and achieved better therapeutic effect, which would further promote pancreatic cancer therapy and provide an important foundation for clinical translation in the future. At present, this study has applied for an international PCT patent protection of “a method for inhibiting cancer-associated fibroblasts and normalizing tumor extracellular matrix”, and has been successfully approved for clinical ethical review of “Single-arm trial of hyperbaric oxygen combined with albumin-bound paclitaxel and gemcitabine for neoadjuvant therapy of resectable pancreatic cancer”.
Master student Xiaoxian Wang, Ningbing Ye, doctoral student Chen Xu, Chen Xiao, Zhijie Zhang from College of Life Science & Technology, HUST contributed equally to this work. This work was financially supported by grants from the National Key Research and Development Program of China (2020YFA0211200, 2020YFA0710700, and 2018YFA0208900), the National Science Foundation of China (31972927), the Scientific Research Foundation of HUST (3004170130) and the Program for HUST Academic Frontier Youth Team (2018QYTD01).
This study is another important research achievement of Prof. Zifu Li on improving the mechanical microenvironment of solid tumor and enhancing the antitumor effect of nanomedicine. Prof. Zifu Li has long been engaged in the research of boosting nanomedicine efficacy with hyperbaric oxygen and has carried out systematic studies on the internal mechanisms. Since 2018, Prof. Zifu Li has published a series of articles on Advanced Science, Nano Today and other international authoritative journals, systematically illustrating that hyperbaric oxygen inhibited collagen deposition, enhanced penetration and improved the antitumor effect of Doxil (Advanced Science, 2018), hyperbaric oxygen decreased solid stress, boosted PD-1 antibody delivery and T cell infiltration for augmented immune responses against solid tumors (Advanced Science, 2021), hyperbaric oxygen improved the delivery efficiency of nanomedicine and effectively eradicated cancer stem cells by reducing tumor extracellular matrix (Nano Today, 2021). This study is a further extension of previous work to clarify that hyperbaric oxygen improved the mechanical microenvironment of solid tumor by inhibiting cancer-associated fibroblasts.
Article Link: https://doi.org/10.1016/j.nantod.2022.101458