On August 23, a research paper entitled "Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy" has been published in Nature Communications, which was performed by Professor Xiangliang Yang’ and Professor Lu Gan’s group from National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology.
Cancer stem cells (CSCs) are the main cause of tumor recurrence, metastasis and drug resistance. CSCs targeting therapy is one of the most effective strategies for clinical treatment of tumors. Nanomedicine plays important roles in precise synergistic treatment, enhanced treatment efficacy and reduced toxic side effects in tumors due to unique properties, such as enhanced permeation and retention (EPR) effects, controlled modification, smart responsiveness and multi-drug co-delivery. Nanomedicine usually achieves targeted delivery of antitumor drugs by coupling the targeting molecules. However, different CSCs express different surface markers. How to efficiently deliver drugs to different CSCs remains a big challenge.
In recent years, Prof. Yang’s team has proposed the “five features” principles for targeted delivery of anticancer drug: Long circulation, tumor accumulation, deep penetration, cellular internalization and drug release. The biomimetic nanomedicine combining the unique function of natural biomaterials with the versatility of artificial nanomaterials has attracted widespread attention in drug delivery. Exosomes, extracellular vesicles secreted from cells, are reported to exhibit long circulation, good biocompatibility and low immunogenicity. More importantly, exosomes express specific proteins with tumor homing capacity. However, the construction of exosome biomimetic nanomedicine faces the challenges, such as the relatively lower yield, the loss of functional proteins caused by traditional extrusion and sonication, which affects the tumor targeting capacity.
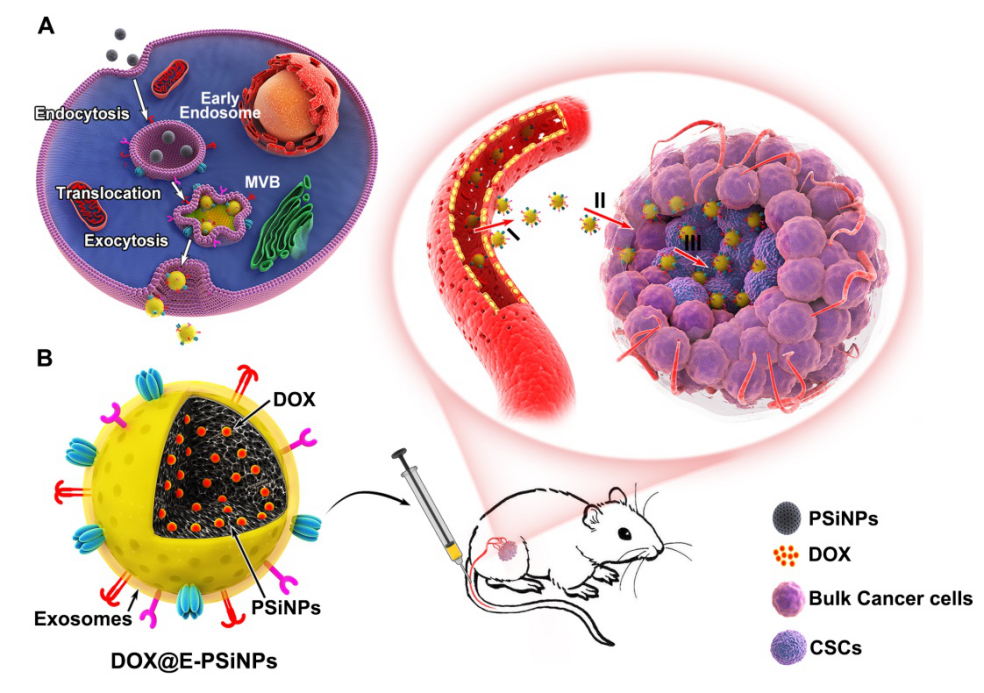
In our study, we constructed exosome-biomimetic porous silicon nanoparticles (E-PSiNPs) exocytosed from tumor cells for CSCs targeting therapy. When tumor cells were incubated with PSiNPs, tumor cells could exocytose the internalized PSiNPs through autophagy. Exosome membrane structure with the thickness of 20 nm was coated around the exocytosed PSiNPs by immunofluorescence, western blot and so on. Compared with traditional exosome purification method, the yield of exosomes was improved by about 34 times and the functional proteins of exosomes were reserved in E-PSiNPs. E-PSiNPs could target bulk tumor cells and CSCs efficiently. Importantly, doxorubicin loaded E-PSiNPs (DOX@E-PSiNPs) exhibited cross-reactive cellular internalization and cytotoxicity in bulk cancer cells and CSCs, which can solve the problem that targeting different CSCs needs different targeting molecular. DOX@E-PSiNPs also could efficiently accumulate in tumor tissues, extravasate from tumor vessels and deeply penetrate into tumor tissues. All these behaviors were related with CD54, an adhesion molecule expressed on E-PSiNPs. Based on these unique characterization, DOX@E-PSiNPs achieved remarkable accumulation in bulk cancer cells and CSCs in tumor tissues, exhibiting excellent antitumor effects and CSCs cytotoxicity in subcutaneous liver tumor model, orthotopic breast tumor model and melanoma lung metastasis tumor model. This study provides new sights for the application of tumor cell-derived exosome biomimetic nanoparticles in tumor therapy.
Profs. Xiangliang Yang and Lu Gan from Huazhong University of Science and Technology, and Prof. Helder A. Santos from University of Helsinki, Finland, are the co-corresponding authors. Postdoctoral fellow Tuying Yong, PhD candidates Xiaoqiong Zhang, Nana Bie from Huazhong University of Science and Technology, and Dr. Hongbo Zhang from Ebo Academic University in Finland are co-first authors. The work was funded by the National Key R&D Programs and the National Natural Science Foundations.