On December 10th, 2020, Professor Liu Jianfeng’s group from college of Life Science and Technology of Huazhong University of Science and Technology published a paper with the title of “Regulation of photosensation by hydrogen peroxide and antioxidants in C. elegans” online on the international well-known journal PLoS Genetics. This study revealed the regulation mechanism of photoreceptor LITE-1 in C.elegans. Zhang Wenyuan and He Feiteng, PhD students of college of life science and technology, are co-first authors. Professor Liu Jianfeng and Professor Shawn Xu from University of Michigan are co-corresponding authors, and Huazhong University of science and technology is the is the first institute contributing for this paper.
Link to the article: (https://doi.org/10.1371/journal.pgen.1009257).
Light sensation is a universal phenomenon found in most organisms. Animals living in dark environments without light-sensing organs are generally believed to have not evolved or to have lost sensitivity to light during evolution. Otherwise, there must be a mechanism(s) that acts to keep such animals in the dark. The eyeless model organism C. elegans exhibits robust phototaxis behavior in response to short-wavelength light, particularly UV light. Liu Lab found that C. elegans senses light through LITE-1, a unique photoreceptor protein that belongs to the invertebrate taste receptor family, this work published on Cell, 2016.
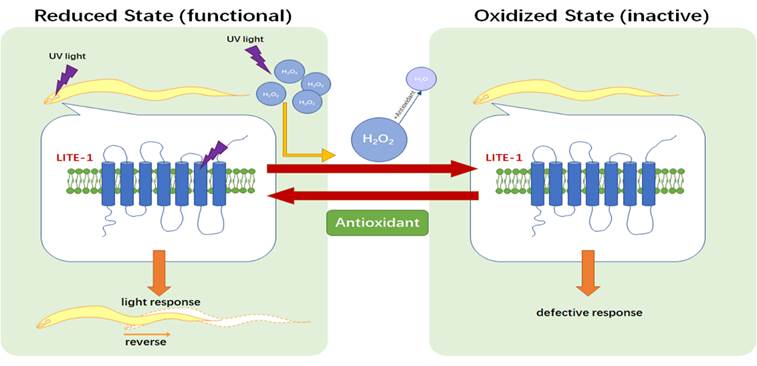
To figure out the mechanism of how LITE-1 regulate photosensation. Here, we performed a forward genetic screen, using light-induced contraction of LITE-1-expressing muscles (i.e. paralysis) as a readout to screen for genes that when mutated suppress LITE-1 function and got six candidates. One major group of lite-1 suppressors are the genes required for producing the two primary antioxidants thioredoxin and glutathione, suggesting that oxidization of LITE-1 inhibits its function. Indeed, the oxidant hydrogen peroxide (H2O2) suppresses phototaxis behavior and inhibits the photoresponse in photoreceptor neurons, whereas other sensory behaviors are relatively less vulnerable to H2O2. Conversely, antioxidants can rescue the phenotype of lite-1 suppressor mutants and promote the photoresponse. As UV light illumination generates H2O2, we propose that upon light activation of LITE-1, light-produced H2O2 then deactivates LITE-1 to terminate the photoresponse, while antioxidants may promote LITE-1’s recovery from its inactive state. Our studies provide a potential mechanism by which H2O2 and antioxidants act synergistically to regulate photosensation in C. elegans.