Recently, a research paper entitled " Hierarchical Vitalization of Oligotyrosine in Mitigating Islet Amyloid Polypeptide Amyloidogenesis through Multivalent Macromolecules with Conformation-Restrained Nanobody Ligands " has been published on the latest issue of ACS Nano , which reported the multivalent macromolecules with conformation-restrained nanobody ligands for the hierarchical vitalization of oligotyrosine in mitigating islet amyloid polypeptide amyloidogenesis. This work is jointly contributed by the teams of Prof. Liang Luo, Prof. Fanling Meng, and Prof. Sheng Wang from College of Life Science & Technology in HUST.
Misfolding and aggregation of human islet amyloid polypeptide (hIAPP) into amyloid fibrils, or the so-called amyloidogenesis, is toxic to pancreatic β cells and widely considered as one of the leading factors to the occurrence of type 2 diabetes. Therefore, the development of inhibitors that can effectively prevent hIAPP aggregation and mitigate hIAPP amyloidogenesis can be used as a potential drug for the treatment of type 2 diabetes. Previously, Luo group reported for the first time that oligotyrosines are intriguing candidates in that they can block hIAPP aggregation through multiplex phenol-hIAPP interactions. However, oligotyrosines containing too many tyrosine units (larger than 3) may fail to inhibit amyloidogenesis due to their increased hydrophobicity and strong self-aggregation propensity. Therefore, it is very necessary to develop a strategy that can enhance the inhibitory efficiency of peptide inhibitors.
Prof. Liang Luo, Fanling Meng and Sheng Wang’s teams developed a strategy to hierarchically vitalize oligotyrosines in mitigating hIAPP amyloidogenesis. Nanobody emerges as an intriguing strategy to vitalize long oligotyrosines, owing to its sequence programmability, conformation specificity, and high binding affinity of its complementary-determining region (CDR) to antigen. Tetratyrosine YYYY (4Y) was grafted into the third complementary-determining region (CDR3) of a parent nanobody to construct a sequence programmed nanobody N4Y, in which the conformation of the grafted 4Y fragment was constrained for a significantly enhanced binding affinity with hIAPP. Next, N4Y was conjugated to N,N-dimethylethylenediamine modified poly(acrylic acid) (PAA-DMA, or PD) to form the multivalent polymer-nanobody conjugate PAA-DMA-N4Y (PDN4Y), which can boost the inhibition effect of 4Y on hIAPP amyloidogenesis through the secondary amplification process through multivalent effect. At very low stoichiometric concentrations, 4Y failed to inhibit hIAPP aggregation, whereas N4Y effectively delayed hIAPP aggregation and partially mitigated hIAPP amyloidogenesis. However, PDN4Y was able to completely inhibit the aggregation and amyloidogenesis of hIAPP, through a synergistic effect of conformation restraint and multivalence.
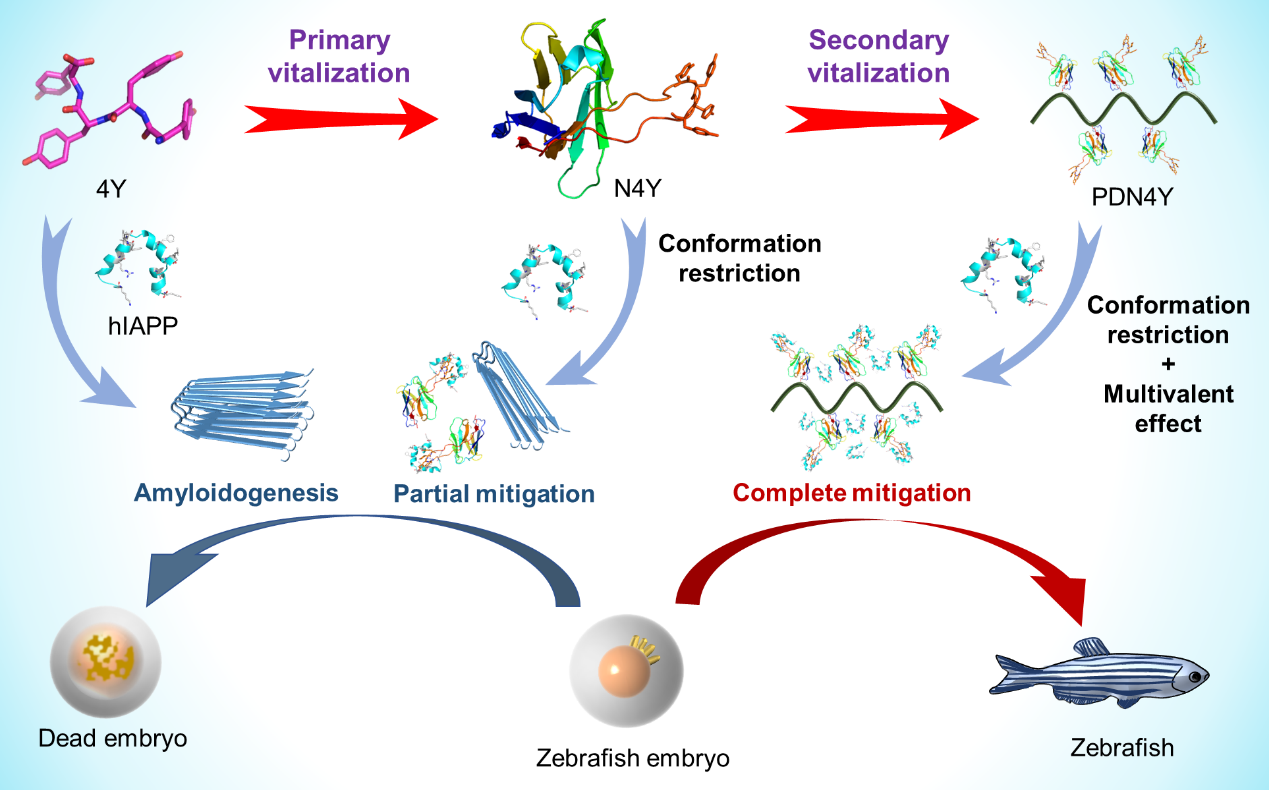
This research successfully developed a hierarchical amplification strategy for oligotyrosines on inhibiting hIAPP amyloidogenes, which not only endows PDN4Y as an effective amyloidogenesis inhibitor with great potential for preclinical and clinical applications, but also provides a universal strategy to progressively amplify the effect of existing peptide-based amyloidogenesis inhibitors.
Ph.D Candidate Yanru Xin and Prof. Sheng Wang from College of Life Science & Technology, HUST, contributed equally to this work. This work was financially supported by grants from the National Natural Science Foundation of China (31900904), the National Basic Research Plan of China (2018YFA0208900), the Natural Science Foundation of Hubei Province (2019CFB483), and the Fundamental Research Funds for the Central Universities, HUST: 2019JYCXJJ034.
URL:https://doi.org/10.1021/acsnano.1c03083