On July 28st, 2021, the team of Prof. Yu Xue from College of Life Science & Technology, Huazhong University of Science collaborated with the team of Prof. Meng-Qiu Dong from National Institute of Biological Sciences, published a research article entitled “Insulin Signaling Regulates Longevity Through Protein Phosphorylation in Caenorhabditis elegans” in Nature Communications. The Dr. Wen-Jun Li, Dr. Chen-Wei Wang, Dr. Li Tao and Yong-Hong Yan are co-first authors of this article. Chen-Wei Wang is the postdoctor of Bioinformatics, College of Life Science & Technology, Huazhong University of Science. Prof. Yu Xue and Prof. Meng-Qiu are the corresponding authors.
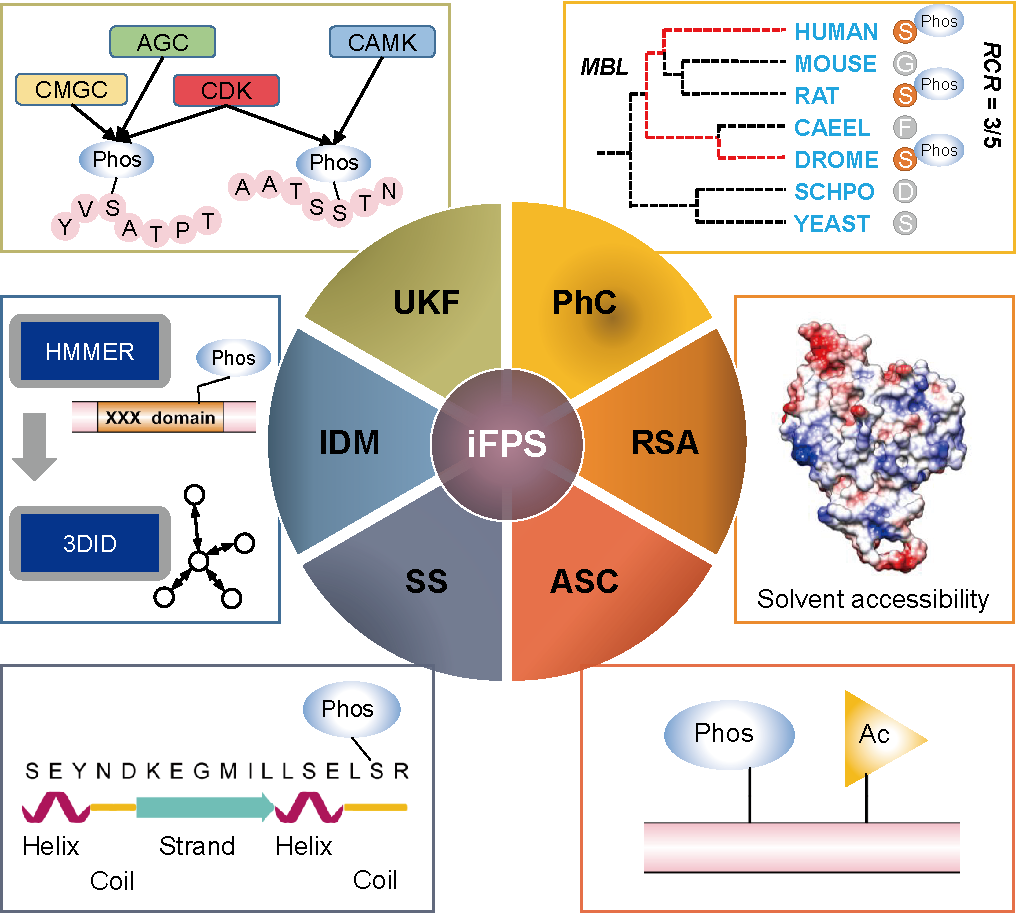
Insulin/IGF-1 Signaling (IIS) is known to constrain longevity by inhibiting the transcription factor FOXO. How phosphorylation mediated by IIS kinases regulates lifespan beyond FOXO remained unclear. In this study, our teams have developed a machine learning-based method named inference of functional phosphosites (iFPS). In iFPS, five frequently used sequence and structure features were integrated to evaluate the functionality of a candidate phosphosite, including the number of predicted upstream kinase families (UKFs), the phosphorylation conservation (PhC), acetylation site co-occurrence (ASC) nearby the phosphosite, and predicted relative surface accessibility (RSA) as well as secondary structures (SSs) of the phosphosite. Also, we added another structural feature, the number of interacting domains and/or motifs (IDMs) that harbor phosphorylated residues. From the literature, 121 known worm phosphosites were collected and taken as the positive data set, whereas the negative data set was prepared by randomly selecting samples from other worm phosphosites in dbPAF. The algorithm of multinomial logistic regression was used for feature integration and model training, with an AUC (area under the curve) value of 0.8784 [95% confidence interval (CI) = 0.8408-0.9129] though the 10-fold cross-validation. As a result, 25 potential lifespan-related phosphosites were predicted. We perform validation experiments to show that AKT-1 pT492 inhibits DAF-16/FOXO and compensates the loss of daf-2 function, that EIF-2α pS49 potently inhibits protein synthesis and daf-2 longevity, and that reduced phosphorylation of multiple germline proteins apparently transmits reduced DAF-2 signaling to the soma. In addition, we analyze the kinase-substrate relations and reveal that casein kinase 2 (CK2) subunits negatively regulate lifespan. Our study reveals detailed functional insights into longevity.
This research was funded by Ministry of Science and Technology of China, Beijing Municipal Science and Technology Commission, the Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, Changjiang Scholars Program of China, and the program for HUST Academic Frontier Youth Team.