On Sept. 29th, 2021, the team of Prof. Yu Xue from College of Life Science & Technology, Huazhong University of Science published a research article entitled “CPLM 4.0: an updated database with rich annotations for protein lysine modifications” in Nucleic Acids Research. Weizhi Zhang and Xiaodan Tan are co-first authors of this article. Prof. Yu Xue is the corresponding authors.

Lysine modification is a very important post-translational modification of proteins. By covalently binding small modification molecules to specific lysine sites of substrate proteins, it can affect many biological processes. For example, it was previously found that acetylation and methylation on histones can influence transcriptional regulation and gene expression as “histone codes”. Subsequent studies found that lysine modification on non-histone proteins also has important functions, such as affecting signal transduction, regulating protein activity and protein trafficking. With the progress of identification technology on lysine modification, more and more sites have been identified and more modification types on lysine sites have been found. For example, a new important lysine modification, lactylation, found on histones in 2019, is related to the development of cancer. Including these important lysine modification types and sites in the database will help to have a comprehensive understanding for the study of lysine modification.
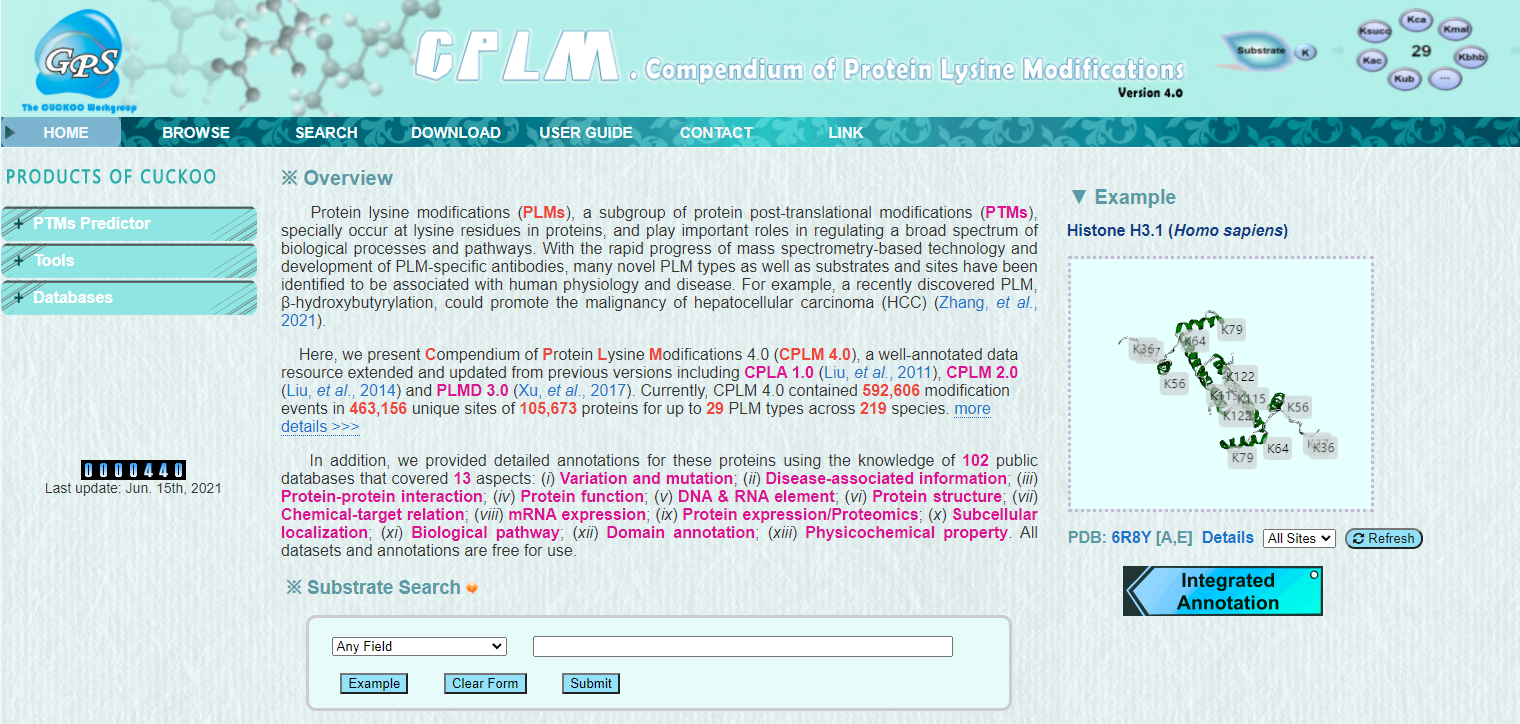
In this work, based on the previous version of PLMD 3.0, we expanded the lysine modification events from 284,780 to 592,606 by manual curation of the literature data and integrating 8 post-translational modification databases. At the same time, we newly collected 9 lysine modification types. So far, CPLM 4.0 integrates 592,606 lysine modification events of 29 lysine modification types within 219 species. We also integrated 105 public databases to make detailed annotations for lysine modification proteins, so as to provide researchers with higher dimensional and more comprehensive information and interpretation for these proteins.
The linkage of article: https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkab849/6377396?searchresult=1
This research is supported by Natural Science Foundation of China (31930021 and 31970633), Fundamental Research Funds for the Central Universities (2019kfyRCPY043), and Changjiang Scholars Program of China.