(Correspondent Liu Xin) On June 4th, a research paper entitled "Hyperbaric oxygen boosts PD-1 antibody delivery and T cell infiltration for augmented immune responses against solid tumors" has been published on Advanced Sciences. This work is contributed by the team of Prof. Zifu Li and Prof. Xiangliang Yang from College of Life Science & Technology, HUST and National Engineering Research Center for Nanomedicine, together with academician Xiao-ping Chen and Prof. Bi-xiang Zhang from Tongji Hospital, HUST.
Cancer immunotherapy, especially immune checkpoint blockade therapy, has attracted extensive attention in recent years. PD-1/PD-L1, a kind of important immune checkpoint, is used by tumor cells to induce T cell dysfunction, so as to escape the immune clearance. To date, FDA has approved PD-1 antibodies including Keytruda from Merck, Opdivo from BMS, Libtayo from Sanofi and Regeneron, Camerlizumab from Jiangsu Hengrui Medicine. However, the clinical application of these PD-1 antibodies has encountered a huge bottleneck: the overall response rate is low, especially in hepatocellular carcinoma, triple negative breast cancer, pancreatic ductal adenocarcinoma and other malignant solid tumors. During the occurrence and development of these malignant solid tumors, due to the rapid proliferation of tumor cells, incomplete development and uneven distribution of vascular system, the internal oxygen supply is insufficient, resulting in tumor hypoxia and high expression of HIF-1α. Tumor hypoxia can further promote tumor development, invasion and metastasis, regulate tumor mechanical microenvironment and immune microenvironment, reduce the response of tumor cells to immunotherapy, leading to the failure of treatment. Therefore, improving tumor hypoxia is of great significance to enhance the effect of cancer immunotherapy. Hyperbaric oxygen therapy (HBO) refers to pure oxygen therapy intermittently given to patients under conditions higher than normal atmospheric pressure, which can effectively relieve tissue hypoxia. In view of this, this study creatively combined hyperbaric oxygen with immune checkpoint blocker PD-1 antibody to treat solid tumors, and developed a new strategy to energize PD-1 antibody immunotherapy by HBO. In this study, the effectiveness, safety and universality of HBO enhancing PD-1 antibody tumor immunotherapy were verified by a variety of mouse models including H22 subcutaneous tumor, H22 orthotopic tumor, Panc02 orthotopic tumor, 4T1 orthotopic tumor and clinical samples of hepatocellular carcinoma. Combining the results from transcriptome and various rodent tumor models, it is confirmed that HBO can significantly relieve the hypoxia of solid tumor, reduce the content of collagen and fibronectin in extracellular matrix, effectively improve the mechanical microenvironment of solid tumor, promote the penetration and enrichment of PD-1 antibody in solid tumors, and increase the infiltration of CD8+ T cells into tumor parenchyma. HBO combined with PD-1 antibody can also reduce the accumulation of MDSCs in tumor tissues, enhance the proliferation, activation and killing capability of cytotoxic T cells, and promote the transformation of macrophages from M2 type to M1 type, and regulate the expression of a series of cytokines, resulting in the switch of tumor microenvironment from immunosuppressive to immunostimulatory.
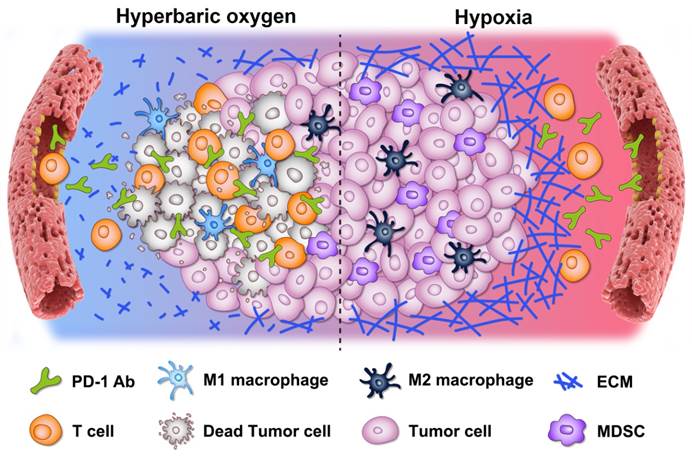
It is worth noting that this study not only verified the effectiveness of HBO and PD-1 antibody combinational therapy in mice, but also demonstrated that HBO can enhance the efficacy of PD-1 antibody in clinical tumor samples by improving the mechanical and immune microenvironment, in the cooperation study with academician Xiao-ping Chen and Prof. Bi-xiang Zhang of Tongji Hospital, HUST. More importantly, the cooperation team has applied the combinational therapy of HBO and PD-1 antibody for clinical trials, and obtained ethical approval for clinical trials. In the next stage, patients with hepatocellular carcinoma will be recruited to carry out clinical trials.
This study creatively combined HBO with PD-1 antibody, and proposed a new strategy to improve the response of immunotherapy. Based on the fact that HBO can improve the tumor mechanical microenvironment and promote the infiltration of lymphocytes in the tumor site, HBO is expected to promote all adoptive cell transfer therapies, which is also the focus of further research of the team in the future.
Post-doctor fellow Xin Liu, master student Ningbing Ye from College of Life Science & Technology, HUST, and PhD candidate Sha Liu from Tongji Hospital, HUST contributed equally to this work. Prof. Zifu Li from College of Life Science & Technology, HUST is the corresponding author. PhD candidate Jiankun Guan, Qingyuan Deng, Zhijie Zhang and Chen Xiao from College of Life Science & Technology, HUST, doctor Ze-yang Ding from Tongji Hospital, HUST participated in this work. This work was assisted by Prof. Xiao-ping Chen and Prof. Bi-xiang Zhang of Tongji Hospital, HUST. This work was financially supported by grants from the National Key Research and Development Program of China (2020YFA0211200, 2020YFA0710700, and 2018YFA0208900), the National Science Foundation of China (31972927 and 31700867), the Scientific Research Foundation of HUST (3004170130), the Program for HUST Academic Frontier Youth Team (2018QYTD01), and the HCP Program for HUST.
Article link: https://doi.org/10.1002/advs.202100233