On April 28, 2021, the teams of Prof. Jianfeng Liu from College of Life Science & Technology, Huazhong University of Science ,key Laboratory of Molecular Biophysics, Ministry of Education and Prof. Yan Zhang, School of Basic Medical Sciences, Zhejiang University published a research article entitled “Structural basis of GABAB receptor-Gi protein coupling”in Nature. In the study, they made a breakthrough in identifying the high-resolution Cryo-electron microscopy structure of class C heterodimer GABAB receptor-G protein complex, revealing a new mode of GPCR conjugation of G protein by dimer GPCR for the first time, which has important guiding significance for the development of novel GPCR allosteric drugs with small toxic and side effects.

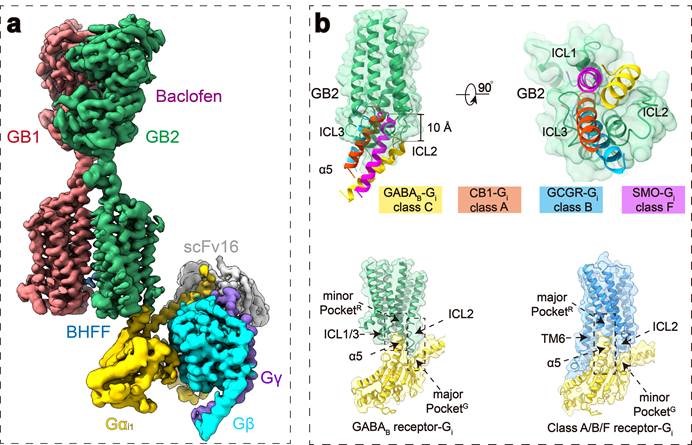
G-protein coupled receptor (GPCR) is the largest membrane receptor protein family in the human body and is closely related to human diseases. GPCRs are essential elements that are involved in cell–cell communication and represent major targets for therapeutic drugs and more than 40% of the drugs on the market are developed with GPCR targets. According to their similarity, GPCR can be divided into four types: A, B, C and F. Recent structural studies have provided important information on how GPCRs can act as nucleotide-exchange factors that allow the release of GDP from the inactive G protein, and then the activation of these proteins upon GTP binding . Several previous structures of activated GPCR–G protein complexes have revealed a similar mode of action for each . Despite differences in the interaction mode of G proteins for various class-A, -B and -F GPCRs, in all previously characterized interactions the C-terminal extremity of the Gα subunit engages with a cavity on the intracellular side of the receptor that results from the opening of this domain owing to the movement of transmembrane helix (TM) 6 relative to TM3. More and more evidence shows that different types of GPCRs can form dimers or polymers. Compared to other classes of GPCRs that can be activated in a monomeric form, class-C GPCRs are mandatory dimers that are composed of two identical or similar subunits. These dimers may activate only one G protein at a time, but the molecular basis of this asymmetric mode of action remains unknown. The elucidation of this mechanism will enrich new molecular pharmacological theories based on GPCR-based dimer and contribute to the development of novel receptor dimer-based high-throughput drug screening methods.
Among the class-C GPCRs that are activated by the neurotransmitter GABA, the GABAB receptor (hereafter referred to as GABAB) is an attractive drug target for the treatment of brain diseases. GABAB is composed of two distinct subunits: GB1 (to which agonists bind) and GB2 (which is responsible for G-protein activation). Each subunit is composed of an extracellular Venus flytrap (VFT) domain and a transmembrane domain (TMD). In this study, we found that, unlike other types of GPCR, TM6 did not outward expand in the transmembrane regions of the two subunits under GABAB receptor activation, only TM3 and TM5 were slightly shifted in the GB2 subunit, leading to changes in the intracellular ring (ICL), where TM3 and the three ICLs formed a shallow groove to bind the G protein. In addition, it has been proved that the binding of G protein with GABAB receptor is a very flexible weak interaction. As shown in the figure, the binding of the GABAB receptor to the G protein is completely different from other types of GPCR, showing the uniqueness of the asymmetric activation of the G protein by the GPCR dimer. The unique binding pattern of GABAB receptor to G protein has also been further verified by numerous functional experiments, and this binding pattern may be conserved in other class-C GPCR dimer. The activated G protein retains all the expected conformational changes of the activated G protein: the α5 helix moves outwards, and the helix domains move relative to each other, opening nucleotide binding sites. The C-terminal of the Gi protein α5 helical is inserted between the long ICL2 and short ICL3 of the GB2 subunit. This binding pattern provides an explanation for the GI/O selectivity of the GABAB receptor, and provides a structural basis for revealing the mechanism of GPCR to produce functional diversity. It was proposed for the first time that steric hindrance between G proteins was the cause of binding only one G protein in GPCR dimer, which solved one of the puzzles in this field.
In 2020, Prof. Jianfeng Liu and Yan Zhang led the world in the analysis of high-resolution cryo-electron microscopy structure of GABAB receptor heterodimer in different states, showing that binding of GABAB receptor agonist causes VFT closure of GB1, which in turn induces rearrangement of TM interface between two subunits. This enables TM to switch from the TM3-TM5/TM3-TM5 interface in the inactive state to the TM6/TM6 interface in the active state (Cell Research., 2020). Based on previous studies, this work further elucidates a new mechanism by which the receptor binds G protein through GB1 and GB2 extracellular interactions, resulting in GB2 "bending" and conformational changes in the GB2 transmembrane region. TM6/TM6 interactions between subunits stabilize the "bending" state of GB2 subunit and thus stabilize receptor activation. This work is a continuation of the previous work, which describes in detail the conformational changes of different domains of GABAB receptor from inactive to activated GPCR-conjugated, and has guiding significance for the study of activation of other GPCR in class -C family