Recently, a research paper entitled " Time‑Programmed Delivery of Sorafenib and Anti‑CD47 Antibody via a Double‑Layer‑Gel Matrix for Postsurgical Treatment of Breast Cancer " has been published on the latest issue of Nano-Micro Letters (IF=16.4), which reported a novel double-layer-gel matrix achieved time-programmed delivery of chemotherapy and immunotherapy drugs for the postsurgical treatment of breast cancer. This work is contributed by the teams of Prof. Liang Luo from College of Life Science & Technology and National Engineering Research Center for Nanomedicine, HUST, and Dr. Chunmeng Sun from China Pharmaceutical University.

The high rate of recurrence metastasis of breast cancer and immunosuppression microenvironment after surgery limited the effects of chemotherapy, radiotherapy, and immunotherapy in breast cancer patients. Due to the plasticity of macrophages, re-educating M2-like tumor-associated macrophages, (TAMs) to anti-tumor M1-like macrophages is promising to reverse immunosuppressive to immune-active microenvironment. Moreover, by blocking the interaction of CD47 with signal regulatory protein alpha (SIRPα), anti-CD47 antibody (aCD47) could promote the macrophages and effector T cells mediated immune response, and enhance the antitumor efficacy. Therefore, this research hypothesized that re-educate TAMs before aCD47 treatment is promising to create an immunogenic microenvironment in the postsurgical treatment model, hence improve the anti-tumor effect.
Prof. Liang Luo and Dr. Chunmeng's teams developed an injectable hierarchically double-layer-gel matrix with soybean phosphatidylcholine (SPC) and glycerol dioleate (GDO), which achieved sequential drug delivery combined with cancer immunotherapy and efficiently prevented tumor recurrence and metastasis in the postsurgical treatment of breast cancer. The outer layer is thermal-responsive and loaded with sorafenib-adsorbed graphene oxide (GO) nanoparticles. GO under manually controlled near-infrared light irradiation can generate mild heat and provoke the release of sorafenib to re-educate TAMs and promote an immunogenic microenvironment. The inner layer loaded with aCD47 can maintain the gel state for a long time, which makes the sustained release of aCD47 block the CD47-SIRPα pathway for a long-term antitumor effect. In vivo 4T1 breast-bearing mouse models demonstrated that the time-programmed multi-drug sequential delivery blocked the CD47 mediated immune escape, efficiently prevented postoperative tumor recurrence and metastasis by synergistically reversing the local immunosuppression and boosting the systemic immune responses.
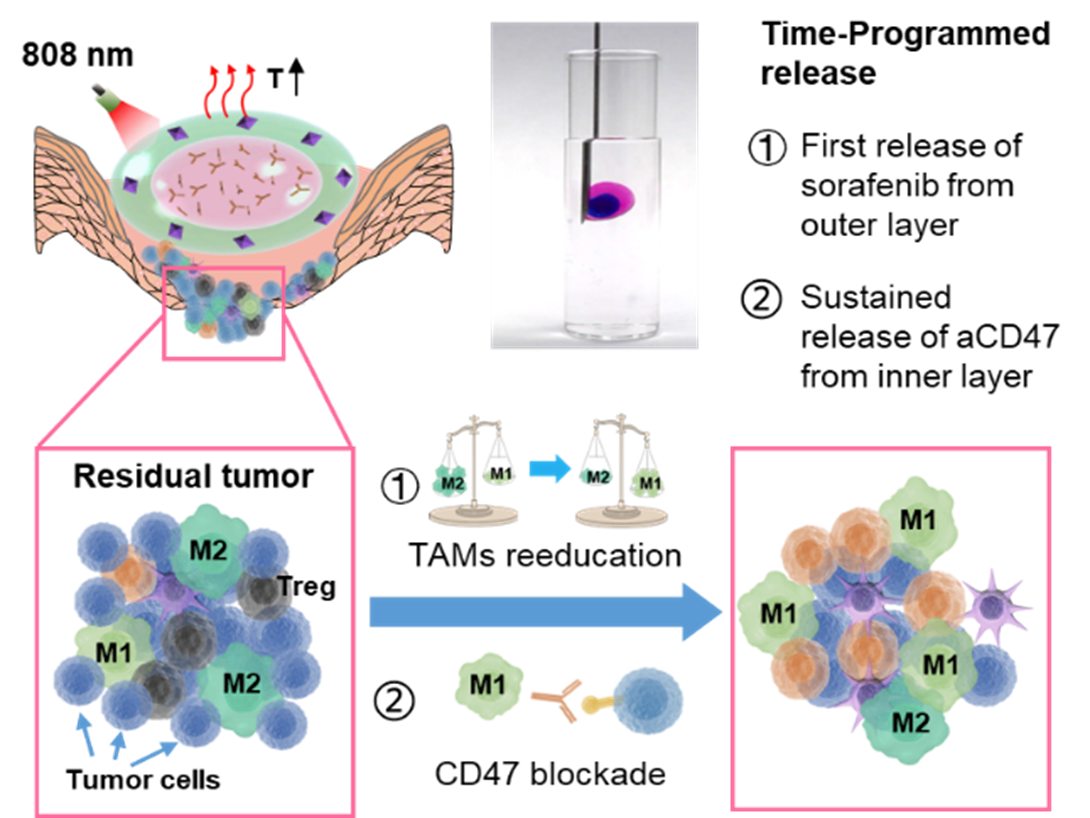
This research developed a novel hierarchical double-layer-gel matrix that achieved local time-programmed multi-drugs sequential delivery, confirmed that an appropriate release dosage of sorafenib could re-educate TAMs and enhance the efficacy of immunotherapy in the postsurgical treatment, it also provides a new way to improve clinical immunotherapy efficacy.
Dr. Liping Huang and master student Yiyi Zhang from College of Life Science & Technology, HUST, and Dr. Yanan Li from China Pharmaceutical University contributed equally to this work. This work was financially supported by grants from the National Key Research and Development Program of China (2018YFA0208903) and the Start-up Funding for Talents Researcher in HUST.
URL:https://doi.org/10.1007/s40820-021-00647-x