(通讯员 陈佶棠)7月25日,美国化学会旗下纳米领域权威期刊《纳米快报》(Nano Letters)刊发了华中科技大学生命科学与技术学院、国家纳米药物工程技术研究中心杨祥良教授与李子福教授课题组题为《一种谷胱甘肽刺激可激活的诊疗一体化纳米药物用于肿瘤的双模成像以及化疗光热综合治疗》(“A Simple Glutathione-Responsive Turn-on Theranostic Nanoparticle for Dual-Modal Imaging and Chemo-Photothermal Combination Therapy”)的研究论文。
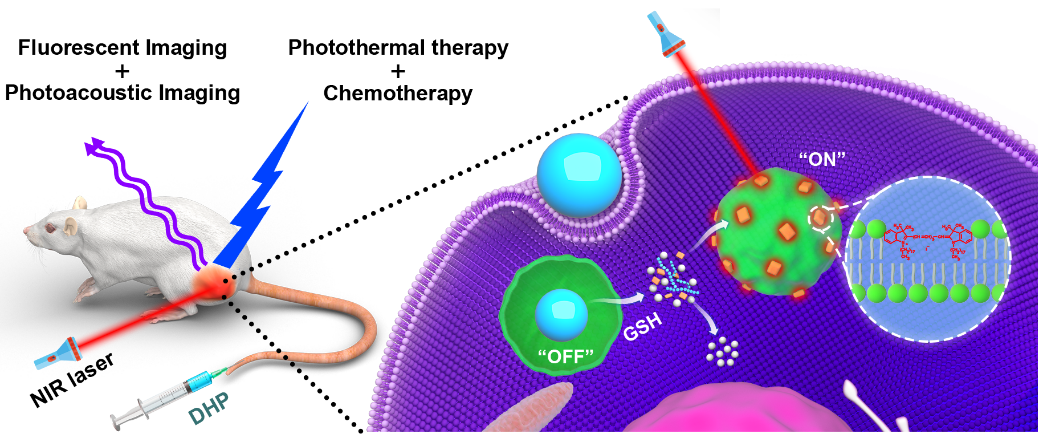
肿瘤微环境刺激可激活的诊疗一体化纳米药物在精准纳米医药中地位十分重要,它们具有信噪比高、背景干扰小等优点,为肿瘤的治疗以及多模式成像诊断提供了一种便捷手段。然而,复杂的成分以及制备工艺严重阻碍了肿瘤微环境刺激可激活诊疗一体化纳米药物的临床转化。本文报道了一种简单的谷胱甘肽刺激可激活的诊疗一体化纳米药物用于肿瘤的双模成像以及化疗光热综合治疗。基于简单的一步透析法制备出只有两种组分(羟乙基淀粉-紫杉醇偶联物,HES-SS-PTX,和近红外荧光分子DiR)的诊疗一体化纳米药物(DHP),巧妙的利用了荧光分子DiR聚集诱导淬灭(ACQ效应)实现肿瘤细胞微环境响应刺激激活。DiR被包裹在HES-SS-PTX形成的疏水核内,DiR分子的荧光因为聚集而淬灭(ACQ效应)。一旦DHP被肿瘤细胞摄取,HES-SS-PTX的二硫键可被肿瘤细胞内GSH裂解,导致偶联的PTX和包载的DiR同步释放。释放的PTX可以发挥其杀肿瘤作用,而DiR会吸附到邻近的内体/溶酶体膜上并恢复其荧光。因此,DHP可以通过DiR的荧光恢复来监测PTX的释放以及治疗效果。DHP还可作为光声活体成像的对比剂,实现对肿瘤深部血管中DHP渗透到肿瘤组织的监控。此外,DHP不仅是一种良好的荧光/光声双模式成像对比剂,同时还能通过化疗光热综合治疗有效的抑制肿瘤生长。更重要的是,监测PTX的释放和治疗效果、活体双模成像以及光热治疗不是由多种成分实现,而是由单一组分DiR完成,从而极大的简化了DHP的组分和制备工艺。谷胱甘肽刺激可激活的诊疗一体化纳米药物DHP设计原理巧妙、成分简单、制备方法简便,具有很高的临床转化潜力。这项研究为推进肿瘤微环境刺激可激活的诊疗一体化纳米药物的临床转化提供了新思路。
华中科技大学生命学院博士生李忆卉、硕士生吴毓昕以及博士生陈佶棠为该论文的共同第一作者。生命学院2015级本科生李世友、张蒙蒙、崔黄陈、李甜甜参与了该项研究。生命学院杨祥良教授和李子福教授为论文通讯作者。该研究得到国家重点研究计划(2018YFA0208900,2015CB931802),国家自然科学基金(31700867, 81627901),以及华中科技大学学术前沿青年团队(2018QYTD01)的资助。
羟乙基淀粉(HES)是临床上最常用的血浆扩容剂,它们是一类经羟乙基化取代的支链淀粉,具有良好的水溶性、生物相容性和生物可降解性。杨祥良教授团队自2002年开始研究羟乙基淀粉制备与生产工艺,已获得4个品种(HES 130/0.4原料与制剂以及HES 200/0.5原料与制剂)药物生产许可证,孵化武汉华科大生命科技有限公司专门生产HES原材料(年产逾百吨),相关技术成果获得湖北省科技进步一等奖,发展的相关技术申报中国发明专利14项,其中已授权3项。在中分子量HES系列原料药及制剂产业化基础上,对标纳米药物研究领域的金标准PEG化策略(PEGylation),进一步研发了基于HES的抗肿瘤纳米药物,杨祥良教授、李子福教授课题组以及万影教授近三年来发展了多种药物偶联和包载的HES化策略(HESylation),取得一系列突出成果,在影响因子6分以上杂志发表论文总共11篇,1篇Journal of Controlled Release (2018, 275, 67-77), 4篇ACS Appl. Mater. Interfaces (2016, 8, 30833-30844; 2017, 9, 19125-19230; 2017, 9, 42225-42238; 2017, 9, 10481-10493), 4篇Nanoscale (2018, 10, 10514-10527; 2018, 10, 17265-17274; 2019, 11, 6217-6227; 2019, 11, 6384-6393), 1篇Chemical Engineering Journal (2018, 349, 129-145), 以及1篇Cancers (2019, 11, 207)。杨祥良教授团队在羟乙基淀粉方向上已经形成独具特色产学研紧密结合的发展模式。
(Reporter Jitang Chen) On July 25th, a research entitled “A Simple Glutathione-Responsive Turn-on Theranostic Nanoparticle for Dual-Modal Imaging and Chemo-Photothermal Combination Therapy” has been published on Nano Letters (American Chemical Society). This work is contributed by the team of Prof. Xiangliang Yang and Prof. Zifu Li from College of Life Science & Technology, HUST and National Engineering Research Center for Nanomedicine.
Tumor microenvironment stimuli activatable theranostic nanoparticles are highly pursued for precise nanomedicine. The theranostic systems have the advantages of a higher signal-to-noise (S/N) ratio and lower background interference, providing a convenient means to simultaneously exert therapeutic effects and realize multimodal imaging for diagnosis. Nonetheless, the multiple components complicate the preparation process and impede the clinical translation. Herein, based on DiR and HES-SS-PTX, this work reported a novel and simple glutathione-responsive turn-on theranostic nanoparticle, DHP, for dual-modal imaging and combination therapy. DHP is prepared with a simple one-step dialysis method. Due to the high concentration in a limited space of the hydrophobic core, the fluorescence of DiR was significantly quenched by the ACQ effect. Nonetheless, once DHP is internalized by cancer cells, the disulfide bond of HES- SS-PTX can be cleaved by endogenous GSH, leading to the simultaneous release of conjugated PTX and loaded DiR. The released PTX could exert its therapeutic effect, while DiR could adsorb onto nearby endosomes/lysosomes membranes and regain its fluorescence. Thus, DHP could monitor the release and therapeutic effect of PTX through the fluorescence recovery of DiR. DHP can also be used as an in vivo probe for both fluorescent and photoacoustic imaging and at the same time achieves a potent antitumor efficacy through chemo-photothermal combination therapy. Importantly, monitoring PTX release and therapeutic action, dual-modal imaging, and photothermal therapy is not achieved from various constituents but realized with single component DiR, thereby greatly simplifying the components and preparations of theranostic nanoparticles. The GSH-responsive turn-on theranostic DHP, prepared with simple components and preparation method, possesses a great clinical translation potential.

PhD candidate YiHui Li, master student Yuxin Wu, PhD candidate Jitang Chen from College of Life Science & Technology, HUST contributed equally to this work. Shiyou Li, MengMeng Zhang, Huangchen Cui, Tiantian Li (undergraduates enrolled in 2015, College of Life Science & Technology, HUST) participated in this work. Prof. Zifu Li and Prof. Xiangliang Yang from College of Life Science & Technology, HUST are corresponding authors. This work was financially supported by grants from National Key Research and Development Program of China (2018YFA0208900), National Basic Research Program of China (2015CB931802), National Science Foundation of China (31700867 and 81627901), and Program for HUST Academic Frontier Youth Team (2018QYTD01).
Derived from waxy maize, which contains more than 95% of amylopectin, hydroxyethyl starch (HES) is therefore highly water soluble with good biocompatibility and biodegradability and has wide clinical use as plasma volume expander. The team of Prof. Xiangliang Yang has been studying the preparation and preparation process of HES since 2002 and has obtained four drug production licenses (raw materials and formulation of HES 130/0.4 and HES 200/0.5). They establish Wuhan HUST Life Science & Technology Co., Ltd., to specialize in producing raw materials of HES (annual output exceeds 100 tons). Based on the HES related technologies, they have won the First Prize for Scientific and Technological Progress in Hubei Province and applied for fourteen Chinese invention patents with three granted. They further develop HES related antitumor nanomedicine. Prof. Xiangliang Yang, Prof. Zifu Li and Prof. Ying Wan have developed several nanoparticles based on HESylation strategy and make a series of outstanding achievements during the past three years. They have published eleven papers in journals with impact factors over 6. Journal of Controlled Release (2018, 275, 67-77), ACS Appl. Mater. Interfaces (2016, 8, 30833-30844; 2017, 9, 19125-19230; 2017, 9, 42225-42238; 2017, 9, 10481-10493), Nanoscale (2018, 10, 10514-10527; 2018, 10, 17265-17274; 2019, 11, 6217-6227; 2019, 11, 6384-6393), Chemical Engineering Journal (2018, 349, 129-145), and Cancers (2019, 11, 207). In the field of HES, the team of Prof. Xiangliang Yang has formed a unique development model with close integration of research and development.